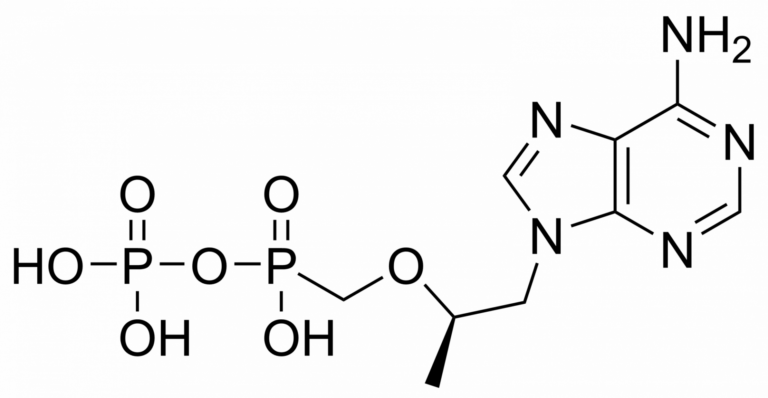
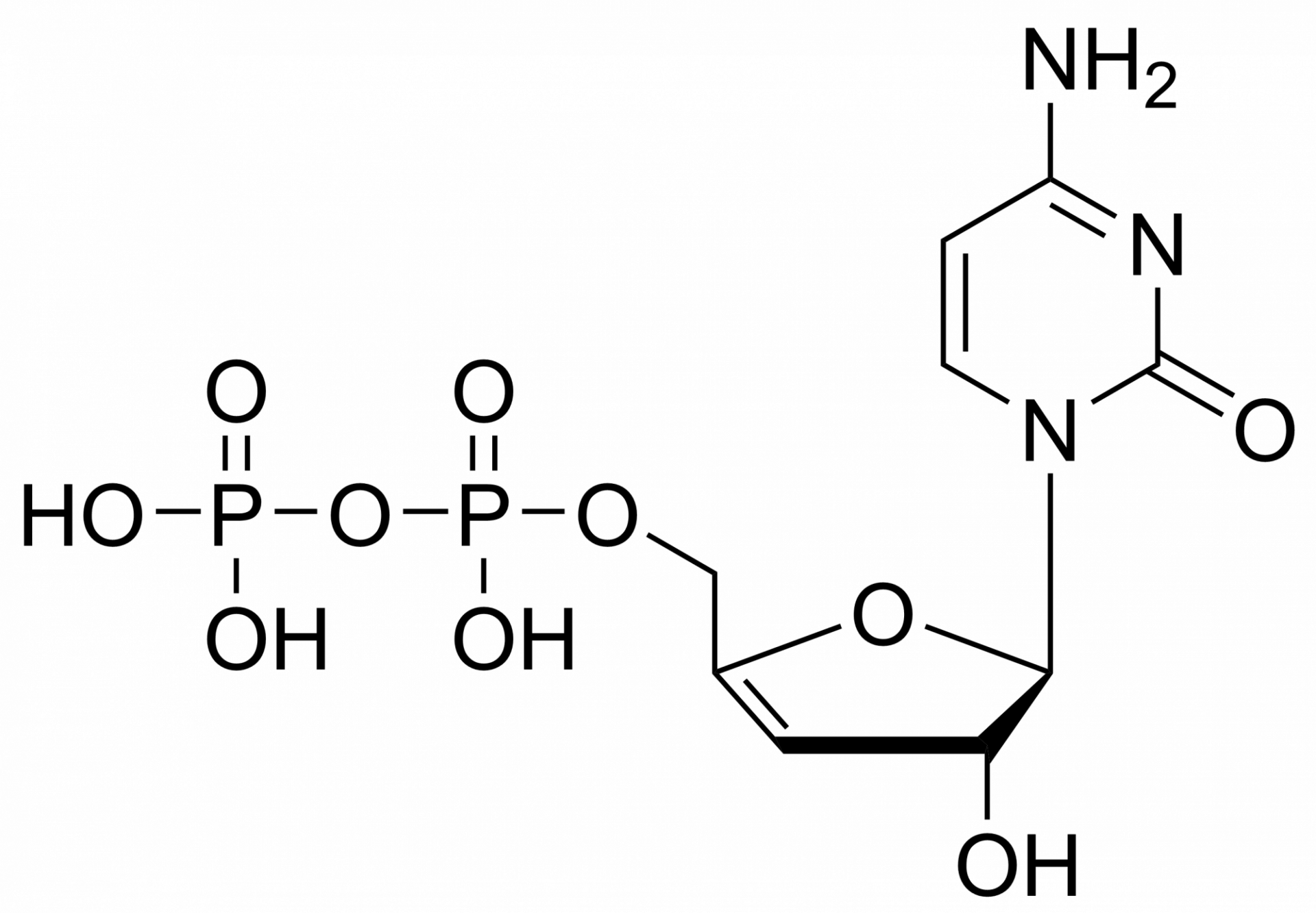
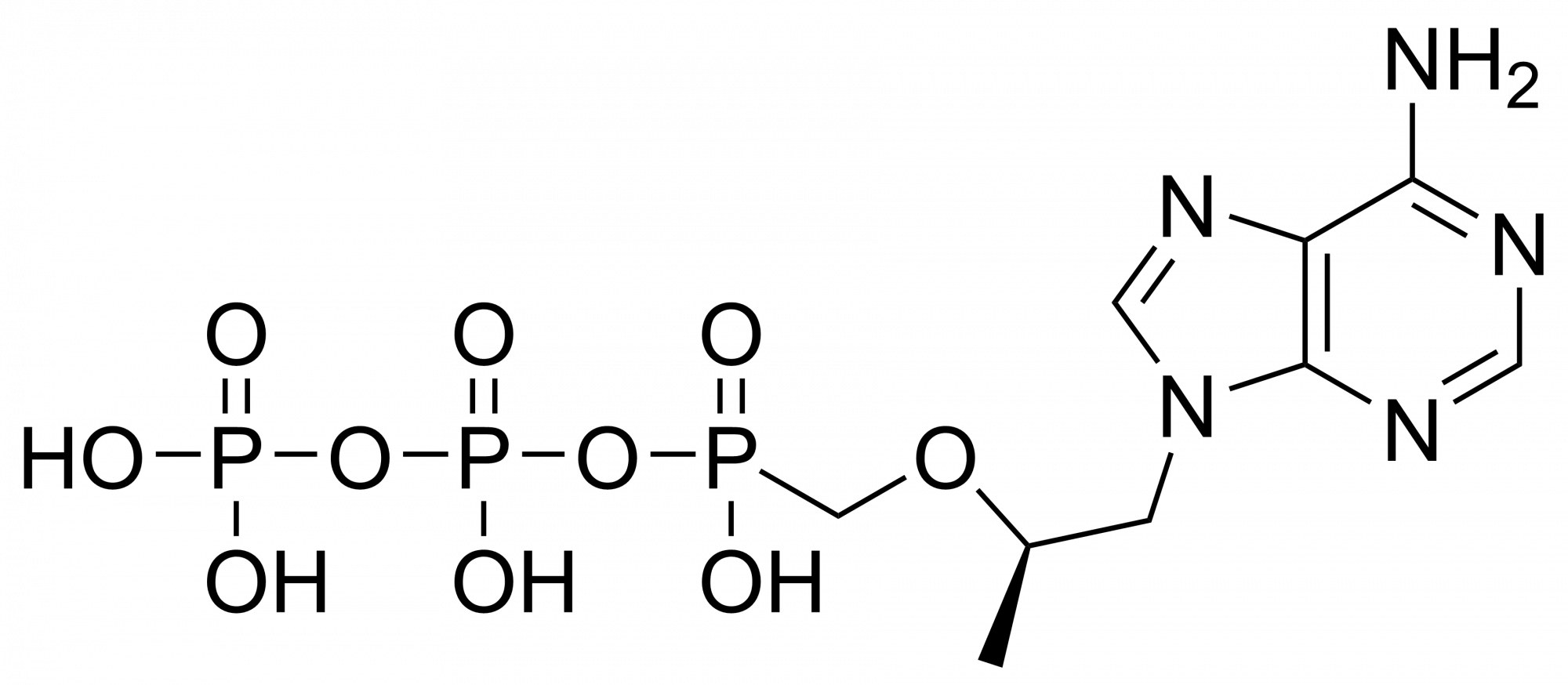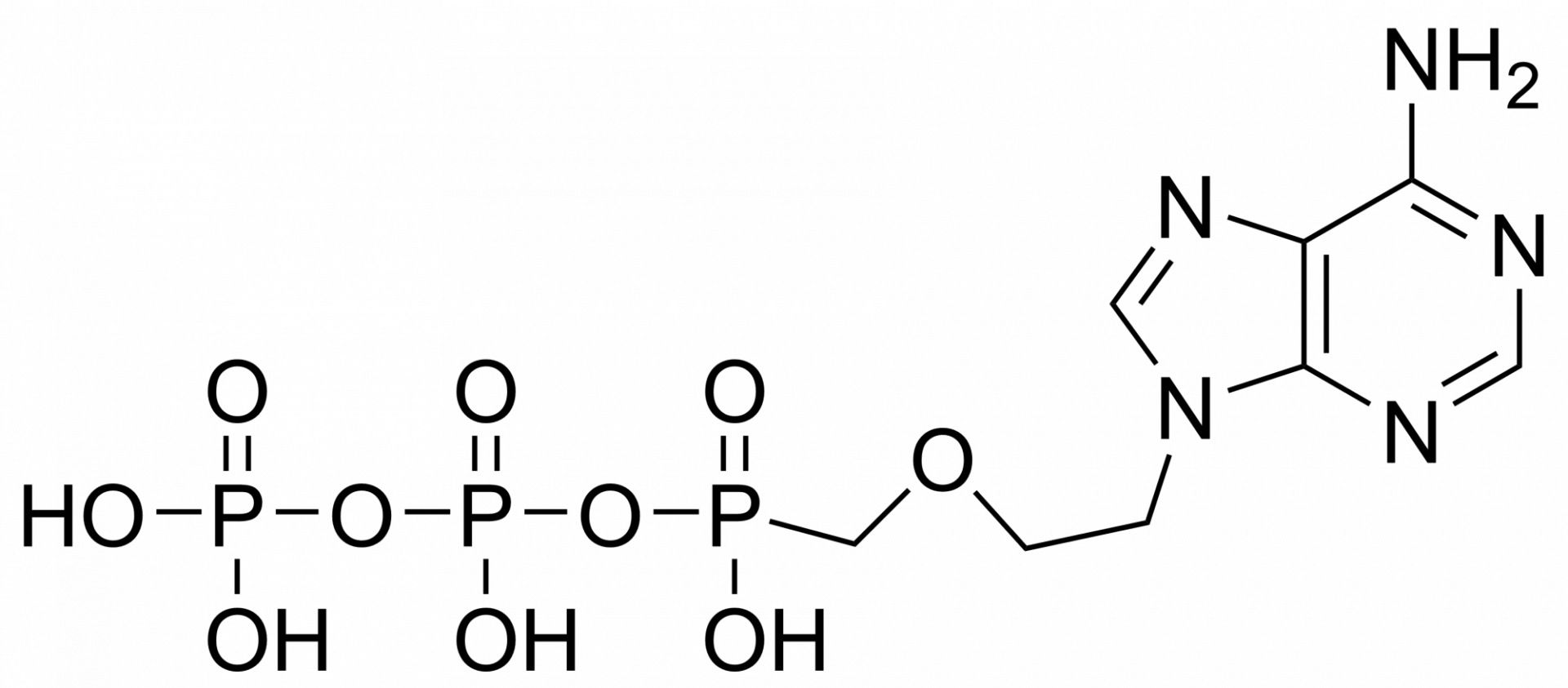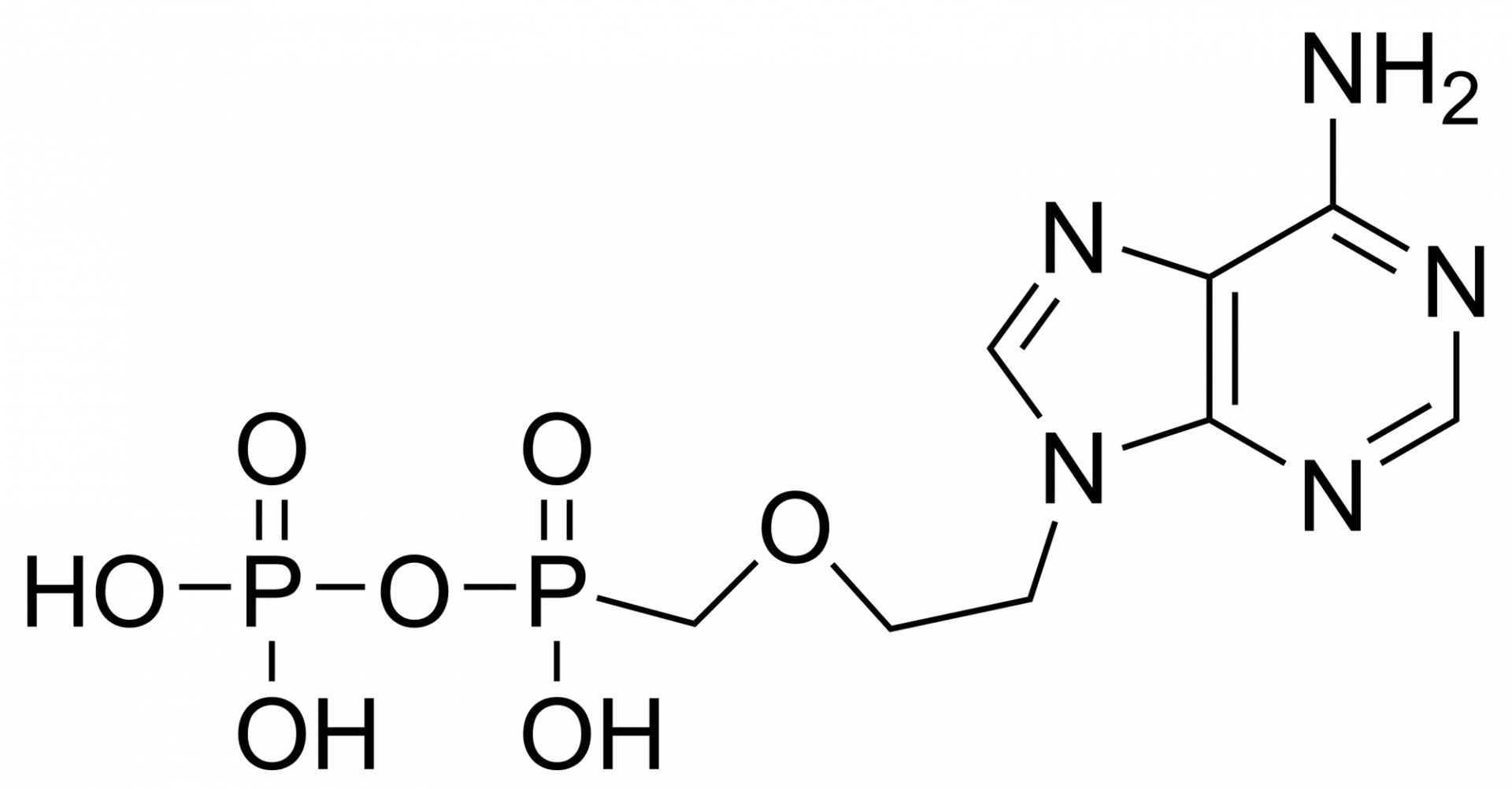Tenofovir monophosphate – CAS 206646-04-0
Tenofovir monophosphate – CAS 206646-04-0 is synthesized by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
min 95%
Package contents
Tenofovir monophosphate triethylammonium salt
This compound is for research use only. We do not sell to patients.
| 1 mg | € 800 | Stock | ||
| 5 mg | € 1500 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Characterisation
Description
Tenofovir monophosphate is a metabolite of tenofovir. Tenofovir is a nucleotide analog reverse-transcriptase inhibitor (NtRTI). It selectively inhibits viral reverse transcriptase, a crucial enzyme in retroviruses such as human immunodeficiency virus (HIV), while showing limited inhibition of human enzymes, such as DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Chemicals are distributed worldwide
Buy Tenofovir monophosphate now, and get your order in 48 hours
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Kearney, B. P., Flaherty, J. F., & Shah, J. (2004). Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clinical pharmacokinetics, 43, 595-612.
- Antoniou, T., Park‐Wyllie, L. Y., & Tseng, A. L. (2003). Tenofovir: a nucleotide analog for the management of human immunodeficiency virus infection. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 23(1), 29-43.
- Louissaint, N. A., Cao, Y. J., Skipper, P. L., Liberman, R. G., Tannenbaum, S. R., Nimmagadda, S., … & Hendrix, C. W. (2013). Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS research and human retroviruses, 29(11), 1443-1450.
- Ray, A. S., Olson, L., & Fridland, A. (2004). Role of purine nucleoside phosphorylase in interactions between 2′, 3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrobial agents and chemotherapy, 48(4), 1089-1095.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors