Favipiravir – CAS 259793-96-9
Favipiravir – CAS 259793-96-9 is provided by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
99 %
Package contents
Favipiravir
This compound is for research use only. We do not sell to patients.
| 100 mg | € 200 | Stock | ||
| 1 g | € 350 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Characterisation
Description
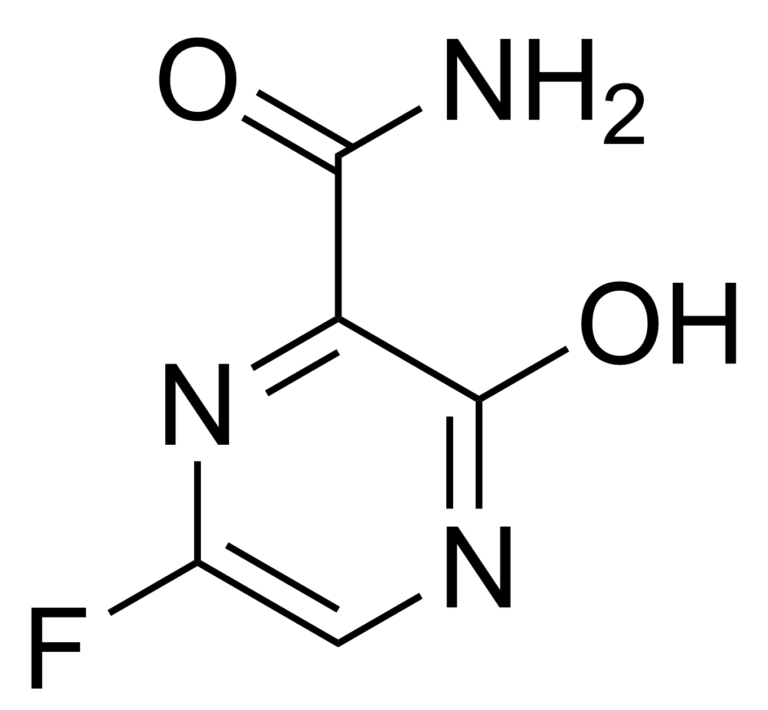
Favipiravir, also known as T-705, or Avigan, is a novel RNA polymerase inhibitor. This antiviral drug has shown activity against many RNA viruses, influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses. Activity against enteroviruses and Rift Valley fever virus has also been proven. Favipiravir does not inhibit RNA or DNA synthesis in mammalian cells and is not toxic to them. In March 2015 was completed a Phase III clinical trial studying the safety and efficacy of Favipiravir in the treatment of influenza. Recently Favipiravir was being studied in China for experimental treatment of COVID-19.
Favipiravir is a pyrazinecarboxamide derivative and it was developed by Toyama Chemical (Fujifilm group) in Japan. This derivative is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5′-triphosphate (favipiravir-RTP). Hypoxanthine guaninephosphoribosyltransferase (HGPRT) is believed to play a key role in this activation process in the human body.
Chemicals are distributed worldwide
Buy Favipiravir now, get your order in 48 hours.
- Shipping through DHL in 48 hours
- All compounds are safely and rigorously packed
Payment
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Shiraki, K.; Daikoku, T., Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacology & Therapeutics 2020, 107512.
- Janowski, A. B.; Dudley, H.; Wang, D., Antiviral activity of ribavirin and favipiravir against human astroviruses. Journal of Clinical Virology 2020, 123, 104247.
- Dong, L.; Hu, S.; Gao, J., Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discoveries & Therapeutics 2020, 14 (1), 58-60.
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020.
- Guo, Q.; Xu, M.; Guo, S.; Zhu, F.; Xie, Y.; Shen, J., The complete synthesis of favipiravir from 2-aminopyrazine. Chemical Papers 2019, 73 (5), 1043-1051.
- Sissoko, D.; Laouenan, C.; Folkesson, E.; M’lebing, A.-B.; Beavogui, A.-H.; Baize, S.; Camara, A.-M.; Maes, P.; Shepherd, S.; Danel, C., Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS medicine 2016, 13 (3).
- Wang, W.; Liu, M.; Xiao, X.; Dai, Q., Synthesis of favipiravir. Journal of International Pharmaceutical Research 2015, (2), 220-224.
- Mentré, F.; Taburet, A.-M.; Guedj, J.; Anglaret, X.; Keïta, S.; de Lamballerie, X.; Malvy, D., Dose regimen of favipiravir for Ebola virus disease. The Lancet Infectious Diseases 2015, 15 (2), 150-151.
- Oestereich, L.; Rieger, T.; Neumann, M.; Bernreuther, C.; Lehmann, M.; Krasemann, S.; Wurr, S.; Emmerich, P.; de Lamballerie, X.; Ölschläger, S., Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. PLoS neglected tropical diseases 2014, 8 (5).
- Oestereich, L.; Lüdtke, A.; Wurr, S.; Rieger, T.; Muñoz-Fontela, C.; Günther, S., Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral research 2014, 105, 17-21.
- Safronetz, D.; Falzarano, D.; Scott, D. P.; Furuta, Y.; Feldmann, H.; Gowen, B. B., Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome. Antimicrobial agents and chemotherapy 2013, 57 (10), 4673-4680.
- Furuta, Y.; Gowen, B. B.; Takahashi, K.; Shiraki, K.; Smee, D. F.; Barnard, D. L., Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral research 2013, 100 (2), 446-454.
- Rocha-Pereira, J.; Jochmans, D.; Dallmeier, K.; Leyssen, P.; Nascimento, M.; Neyts, J., Favipiravir (T-705) inhibits in vitro norovirus replication. Biochemical and biophysical research communications 2012, 424 (4), 777-780.
- Kiso, M.; Takahashi, K.; Sakai-Tagawa, Y.; Shinya, K.; Sakabe, S.; Le, Q. M.; Ozawa, M.; Furuta, Y.; Kawaoka, Y., T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proceedings of the National Academy of Sciences 2010, 107 (2), 882-887.
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D. F.; Barnard, D. L.; Gowen, B. B.; Julander, J. G.; Morrey, J. D., T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral research 2009, 82 (3), 95-102.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors