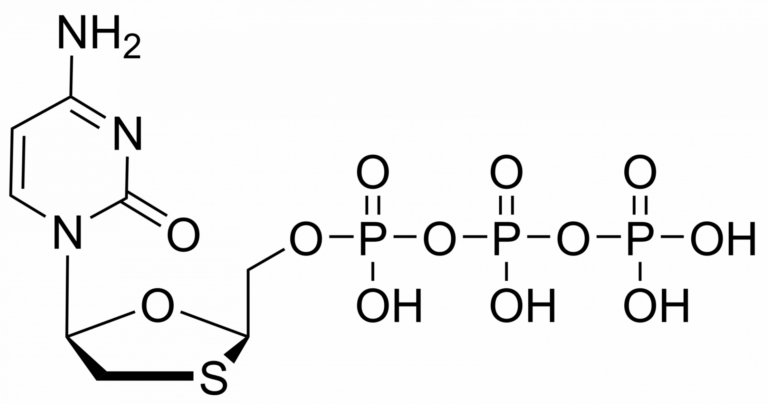
Lamivudine triphosphate – CAS 143188-53-8
Lamivudine triphosphate – CAS 143188-53-8 is synthesised by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
99%+
Package contents
Lamivudine triphosphate Et3NH+ salt
This compound is for research use only. We do not sell to patients.
| 1 mg | € 450 | Stock | ||
| 5 mg | € 1900 | Stock | ||
| 10 mg | € 3500 | Stock | ||
| 100mM/10μL | € 450 | Stock | ||
| 10mM/100μL | € 450 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Stock
Stock
Stock
Characterisation
Description
Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typically used in combination with other antiretrovirals such as zidovudine and abacavir. Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV. Lamivudine is taken by mouth as a liquid or tablet.
Common side effects include nausea, diarrhea, headaches, feeling tired, and cough. Serious side effects include liver disease, lactic acidosis, and worsening hepatitis B among those already infected. It is safe for people over three months of age and can be used during pregnancy. The medication can be taken with or without food. Lamivudine is a nucleoside reverse transcriptase inhibitor and works by blocking the HIV reverse transcriptase and hepatitis B virus polymerase.
Lamivudine was patented in 1995 and approved for use in the United States in 1995. It is on the World Health Organization’s List of Essential Medicines. It is available as a generic medication.
You can find more information about antiviral drug research at Sigut Labs in our article.
Chemicals are distributed worldwide
Buy Lamivudine triphosphate now, and get your order in 48 hours
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Johnson, M. A., Moore, K. H., Yuen, G. J., Bye, A., & Pakes, G. E. (1999). Clinical pharmacokinetics of lamivudine. Clinical pharmacokinetics, 36, 41-66.
- Lai, C. L., Chien, R. N., Leung, N. W., Chang, T. T., Guan, R., Tai, D. I., … & Gray, D. F. (1998). A one-year trial of lamivudine for chronic hepatitis B. New England Journal of Medicine, 339(2), 61-68.
- Dienstag, J. L., Goldin, R. D., Heathcote, E. J., Hann, H. W. L., Woessner, M., Stephenson, S. L., … & Schiff, E. R. (2003). Histological outcome during long-term lamivudine therapy. Gastroenterology, 124(1), 105-117.
- Perry, C. M., & Faulds, D. (1997). Lamivudine: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs, 53, 657-680.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors