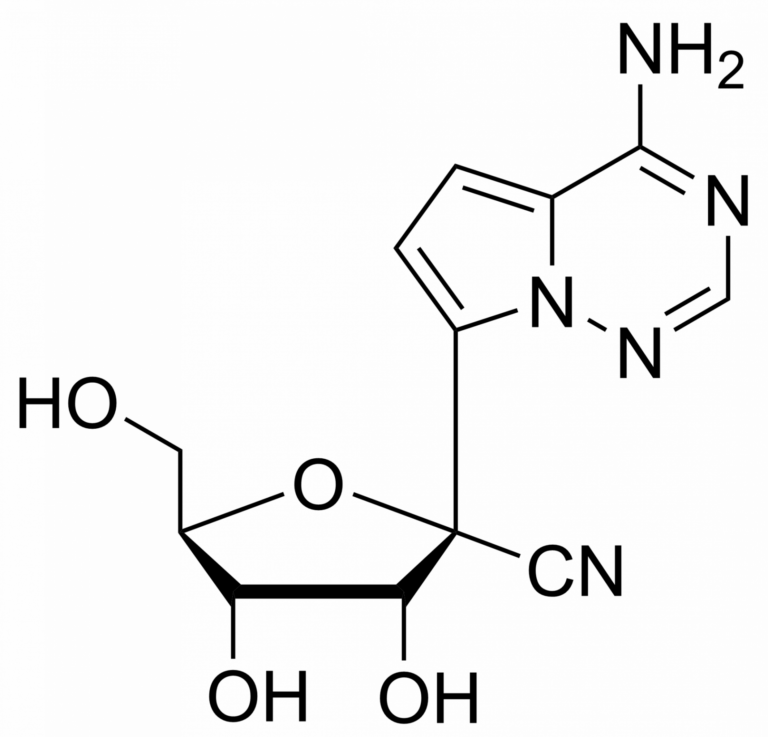
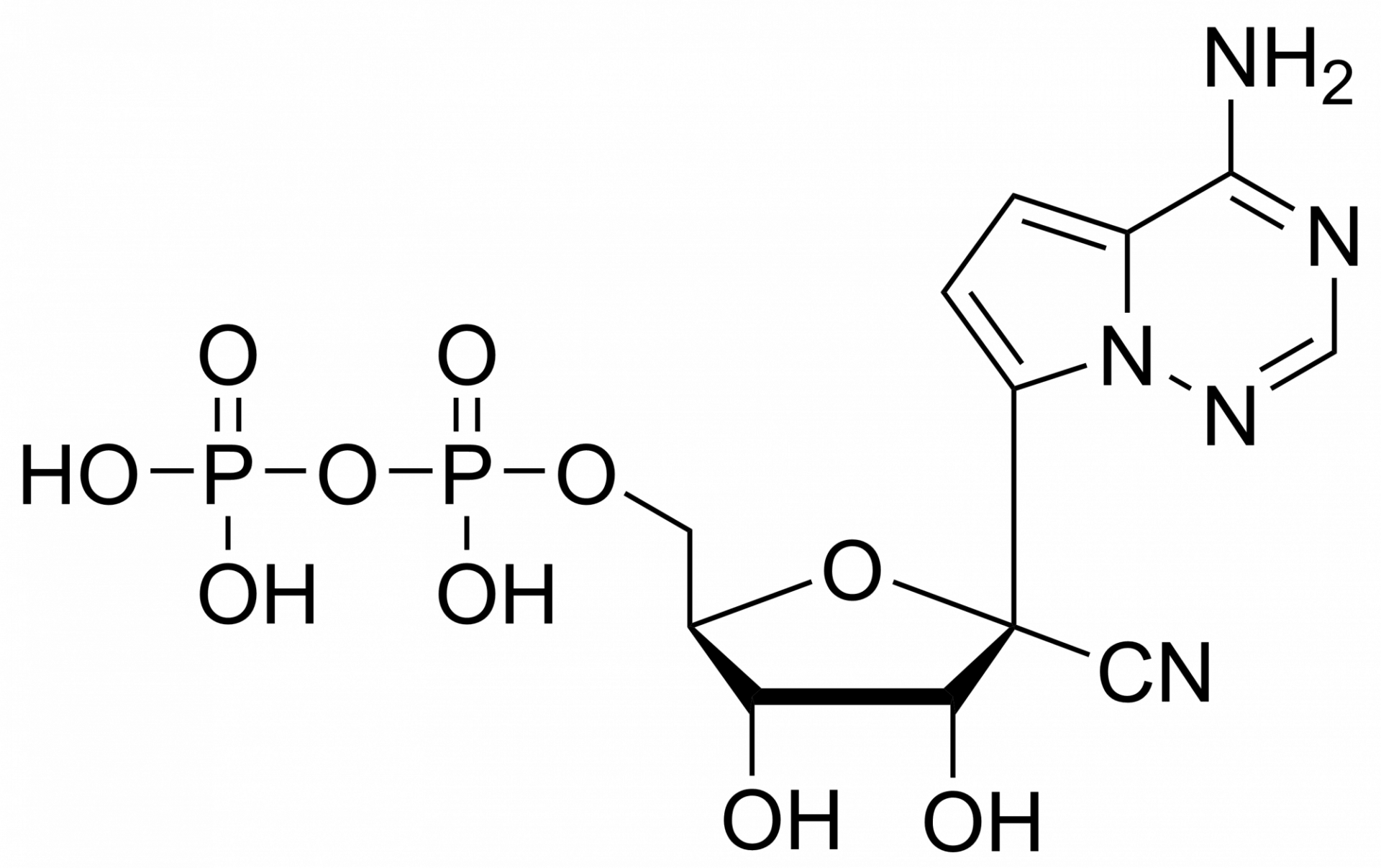
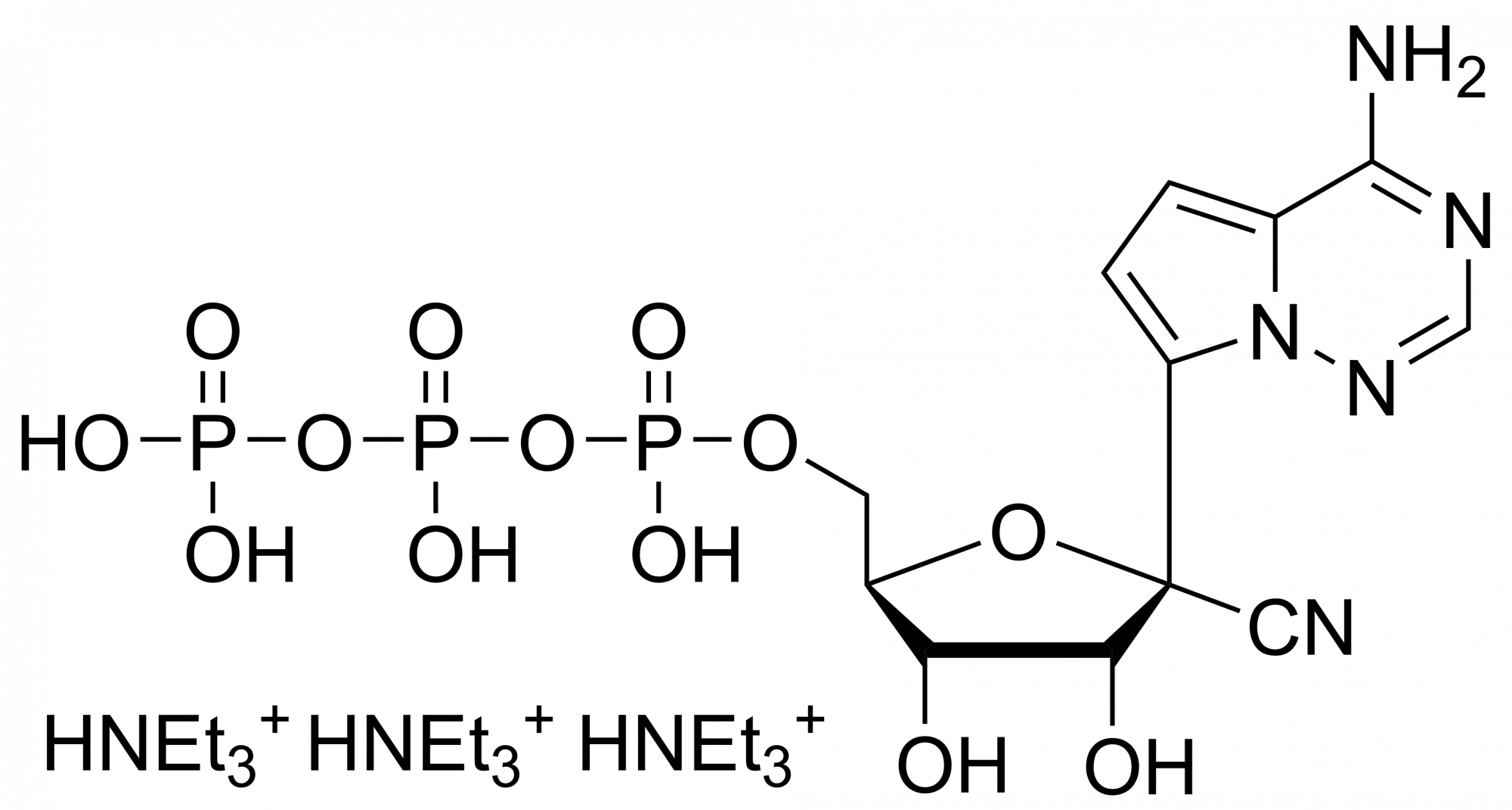
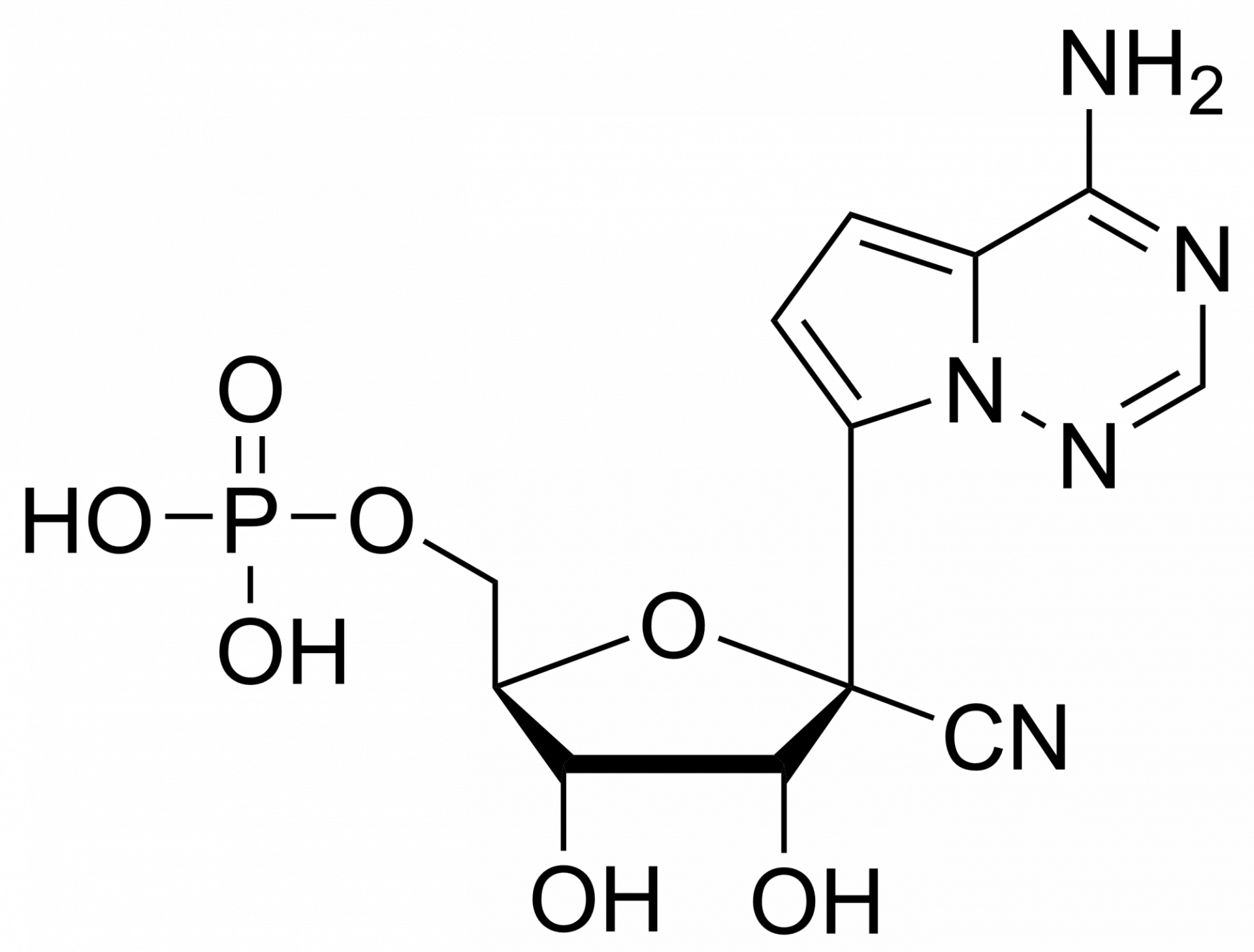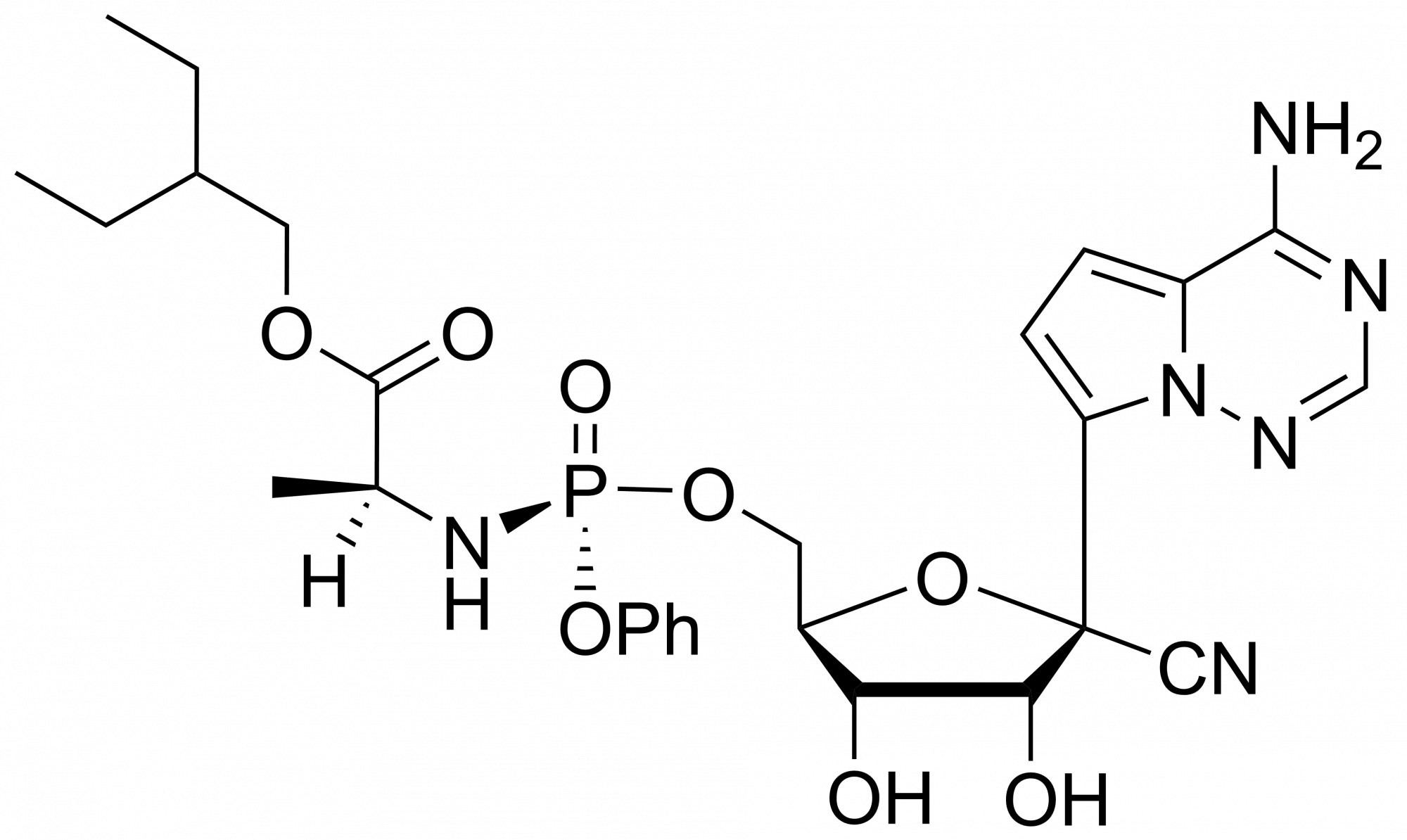GS 441524 – CAS 1191237-69-0
GS 441524 – CAS 1191237-69-0 is provided by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
99 %
Package contents
GS 441524
This compound is for research use only. We do not sell to patients or for veterinary use.
| 10 mg | € 100 | Stock | ||
| 50 mg | € 180 | Stock | ||
| 100 mg | € 250 | Stock | ||
| 1 g | € 600 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Stock
Stock
Characterisation
Description
This nucleoside is an essential building block to the synthesis of Remdesivir triphosphate (1355149-45-9) and Remdesivir (1809249-37-3). Nucleoside derivate is used in the treatment of Feline infectious peritonitis (FIP). This fatal disease affects cats and is caused by a virus from a group of coronavirus. Remdesivir is a nucleoside prodrug that metabolizes into GS-441524 which is phosphorylated to Remdesivir triphosphate. This phosphorus analogue is mainly an active compound in the treatment of Ebola and COVID-19.
We are also offering Remdesivir and Remdesivir triphosphate.
We cannot sell this compound for medical or veterinary use.
Chemicals are distributed worldwide
Buy GS 441524 now, get your order in 48 hours.
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Shannon, A.; Le, N. T.-T.; Selisko, B.; Eydoux, C.; Alvarez, K.; Guillemot, J.-C.; Decroly, E.; Peersen, O.; Ferron, F.; Canard, B., Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020, 178, 104793.
- Ju, J.; Li, X.; Kumar, S.; Jockusch, S.; Chien, M.; Tao, C.; Morozova, I.; Kalachikov, S.; Kirchdoerfer, R. N.; Russo, J. J., Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020, 1-18.
- Jordan, P. C.; Liu, C.; Raynaud, P.; Lo, M. K.; Spiropoulou, C. F.; Symons, J. A.; Beigelman, L.; Deval, J., Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathogens 2018, 14 (2), e1006889/1-e1006889/23.
- Siegel, D.; Hui, H. C.; Doerffler, E.; Clarke, M. O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; Wang, Q.; Wolfe, L.; Jordan, R.; Soloveva, V.; Knox, J.; Perry, J.; Perron, M.; Stray, K. M.; Barauskas, O.; Feng, J. Y.; Xu, Y.; Lee, G.; Rheingold, A. L.; Ray, A. S.; Bannister, R.; Strickley, R.; Swaminathan, S.; Lee, W. A.; Bavari, S.; Cihlar, T.; Lo, M. K.; Warren, T. K.; Mackman, R. L., Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60 (5), 1648-1661.
- Clarke, M. O. N. H.; Jordan, R.; Mackman, R. L.; Ray, A. S.; Siegel, D. Preparation of amino acid-containing nucleosides for treating flaviviridae virus infections. 2017-US28243, 2017184668, 20170419., 2017.
- Warren, T. K.; Jordan, R.; Lo, M. K.; Ray, A. S.; Mackman, R. L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H. C.; Larson, N.; Strickley, R.; Wells, J.; Stuthman, K. S.; Van Tongeren, S. A.; Garza, N. L.; Donnelly, G.; Shurtleff, A. C.; Retterer, C. J.; Gharaibeh, D.; Zamani, R.; Kenny, T.; Eaton, B. P.; Grimes, E.; Welch, L. S.; Gomba, L.; Wilhelmsen, C. L.; Nichols, D. K.; Nuss, J. E.; Nagle, E. R.; Kugelman, J. R.; Palacios, G.; Doerffler, E.; Neville, S.; Carra, E.; Clarke, M. O.; Zhang, L.; Lew, W.; Ross, B.; Wang, Q.; Chun, K.; Wolfe, L.; Babusis, D.; Park, Y.; Stray, K. M.; Trancheva, I.; Feng, J. Y.; Barauskas, O.; Xu, Y.; Wong, P.; Braun, M. R.; Flint, M.; McMullan, L. K.; Chen, S.-S.; Fearns, R.; Swaminathan, S.; Mayers, D. L.; Spiropoulou, C. F.; Lee, W. A.; Nichol, S. T.; Cihlar, T.; Bavari, S., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature (London, United Kingdom) 2016, 531 (7594), 381-385.
- Chun, B. K.; Clarke, M. O. N. H.; Doerffler, E.; Hui, H. C.; Jordan, R.; Mackman, R. L.; Parrish, J. P.; Ray, A. S.; Siegel, D. Preparation of nucleosides and methods for treating Filoviridae virus infections. 2015-14926062 20160122374, 20151029., 2016.
- Cho, A.; Saunders, O. L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J. E.; Feng, J. Y.; Ray, A. S.; Kim, C. U., Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic & Medicinal Chemistry Letters 2012, 22 (8), 2705-2707.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors