Reagent of the month – Polytriazole ligands for the copper catalysed alkyne–azide cycloaddition (CuAAC)
The copper (i) catalysed alkyne–azide cycloaddition (CuAAC) is one of the most frequently used bioconjugation reactions nowadays. It was first reported in 2002 independently by the labs of M. Meldal (https://doi.org/10.1021/jo011148j) and K. B. Sharpless (https://doi.org/10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0).
The original paper from the Meldal lab describes the application of the CuAAC reaction for bioconjugation of protected saccharides to peptides, but application of the original ligand-free CuAAC procedure to more sensitive biomolecules like nucleic acids or to live cells have been limited. The main issues with the ligand-free CuAAC reaction in the early attempts for its biorthogonal application were the generation of reactive oxygen species (ROS) by the Cu (ii)/Cu (i) redox cycle and the toxicity of copper ions resulting from its coordination to amino acids. (https://doi.org/10.1021/ja2083027, https://doi.org/10.3390/molecules21101393).
To overcome these issues, ligand assisted CuAAC protocols have been developed and the field of research of copper ligands for CuAAC is still developing. A second strategy that is widely used for bioconjugations is strain promoted alkyne–azide cycloaddition (SPAAC) which proceeds fast enough even without copper (i) catalysis due to the ring strain relief of the used cyclic alkynes such as bicyclononyne (BCN).
This short article will focus on the former of the above mentioned strategies and will summarize the development and the current best ligands that limit the formation of ROS and the toxicity of Cu (i) ions, as well as considerably speed up the CuAAC reaction.
Types of CuAAC accelerating ligands
The classic ligand-free CuAAC reaction still uses amine bases such as triethylamine or DiPEA which can act as monodentate ligands for the Cu (i) ions, but due to the effect of these amines on the rate of the click reaction is negligible.
Polydentate amine ligands such as hexamethyltriethylenetetraamine (HMTETA), tris[2-(dimethylamino)ethyl]amine (Me6TREN) and pentamethyldiethylenetriamine (PMDTA) increase the rate of the CuAAC reaction significantly when used in 1:1 stoichiometric ratio with the copper (i) species. The rate coefficients for each ligand are given under the structure of each ligand (Figure 1). (https://doi.org/10.1021/ma061592u)
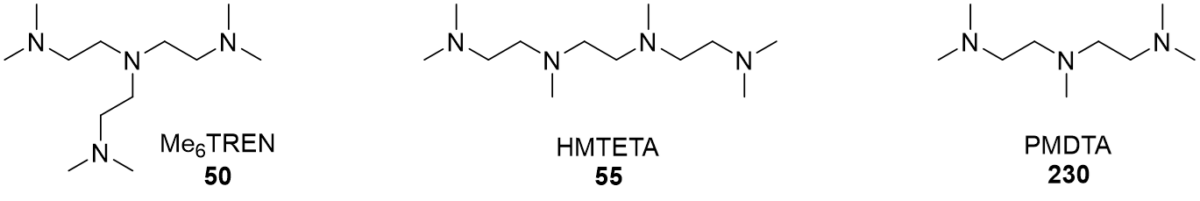
Since the copper (i) complexes are tetrahedral, the best results are achieved with tridentate ligands which occupy three of the four coordination spots and leave one free for the coordination of the alkyne. This can be seen from the relative rates of the CuAAC with PMDTA vs HMTETA as the ligand. The tridentate PMDTA increases the reaction rate about 4 times more than the tetradentate HMTETA.
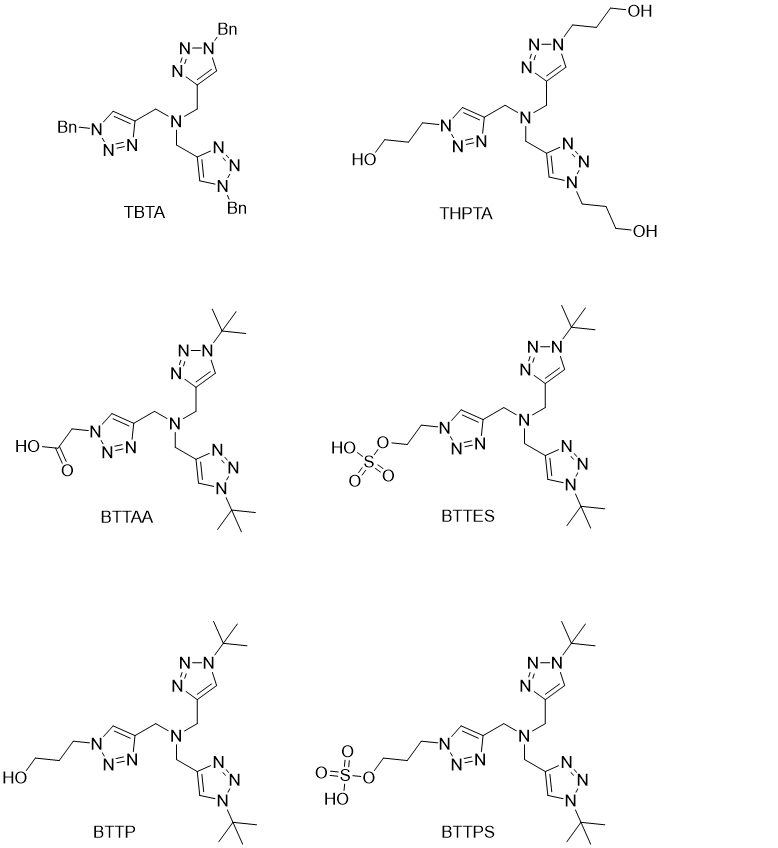
In 2004, first tris-(triazole-ylmethyl)amine ligands were reported by the Fokin group (https://doi.org/10.1021/ol0493094) which have shown even higher increase in the reaction rate and the stabilization of the Cu (i) oxidation state with TBTA showing some of the best results. Optimization of the side chains connected to the triazole rings led to the development of several other tripodal triazole ligands with improved water solubility and Cu (i) stabilization. These include THPTA (https://doi.org/10.1002/anie.200905087), BTTAA (https://doi.org/10.1002/anie.201101817), BTTES (https://doi.org/10.1021/ja106553e), and some of the currently best performing ligands for CuAAC: BTTP and BTTPS (https://doi.org/10.1002/asia.201100385) (Figure 2).
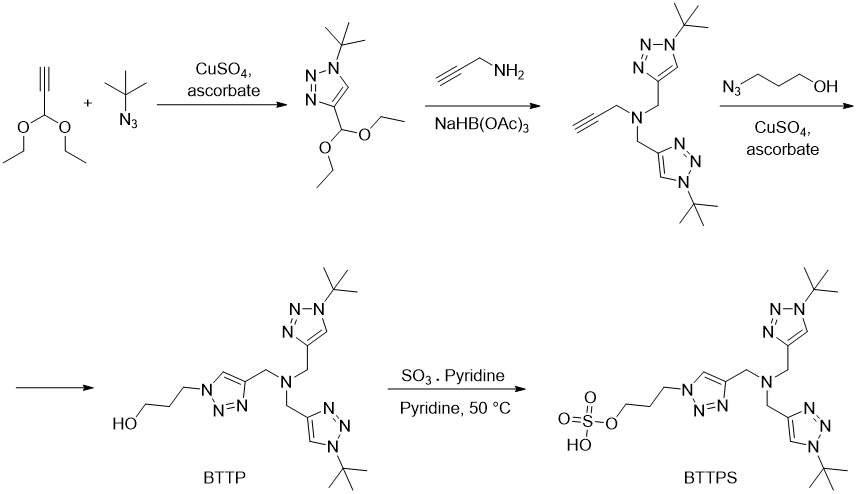
Preparation of BTTP and BTTPS
The preparation of BTTP starts by a CuAAC reaction of tert-butylazide with diethylacetal of propiolaldehyde (3,3-diethoxyprop-1-yn). Two equivalents of the resulting triazole can be used in reductive alkylation of propargylamine and the last step is another CuAAC reaction with 3-azidopropan-1-ol. The corresponding sulphate (BTTPS) can be then prepared by treating BTTP with sulfur trioxide in pyridine (Figure 3). (https://doi.org/10.1002/asia.201100385) BTTES can be prepared similarly using 2-azidoethanol in the last click reaction. (https://doi.org/10.1021/ja106553e)
Application in chemical biology
Ligand assisted CuAAC reaction has been used in many applications in chemical biology for bioconjugation of small molecules to proteins, glycans, lipids or nucleic acids as well as protein–protein coupling. (https://doi.org/10.3390/molecules21101393)
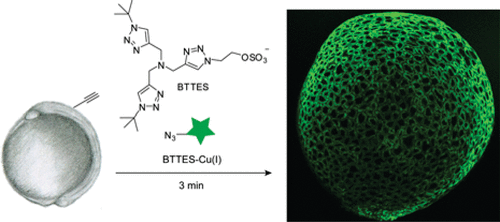
One example is fluorescent labelling of surface glycans on live cells which were first incubated with alkyne derivatives of sugars which the cell incorporates into the surface glycans. In this case, BTTES was used as the ligand (Figure 4). (https://doi.org/10.1021/ja106553e)
OUR CASE STUDY
Scale-up to accelerate drug discovery
Our experience helped overcome development hurdles for potential cancer & mental health drugs.
Read moreEmpowering neuro research with pro-N6pA
Sigut Labs scaled up pro-N6pA production, simplifying AMPylation research & boosting accessibility.
Read moreADC development leaps with new linkers
Novel linker design expedited ADC advancement, leading to promising lead compounds faster.
Read moreLincomycin derivative scale-up
Over 30 g of the desired product with exceptional purity was obtained through our optimized procedure.
Read morePurifying 350 kg of vitamin K2 oil
Our innovative scale-up technology helped to reduce the client’s purification process from days to hours.
Read more
Custom synthesis
Providing for a custom synthesis of previously reported molecules using described synthetic procedures.

Contract research
Developing novel synthetic routes to provide undescribed compounds in organic, bioorganic, and medicinal chemistry.

Scale-Up
Helping you go from lab scales to an industrial scale by applying our cutting-edge instrumentation.

Our Experts


Partners & distributors











