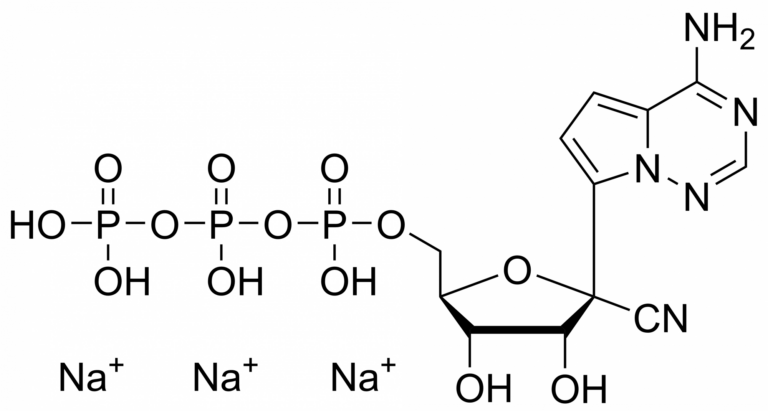
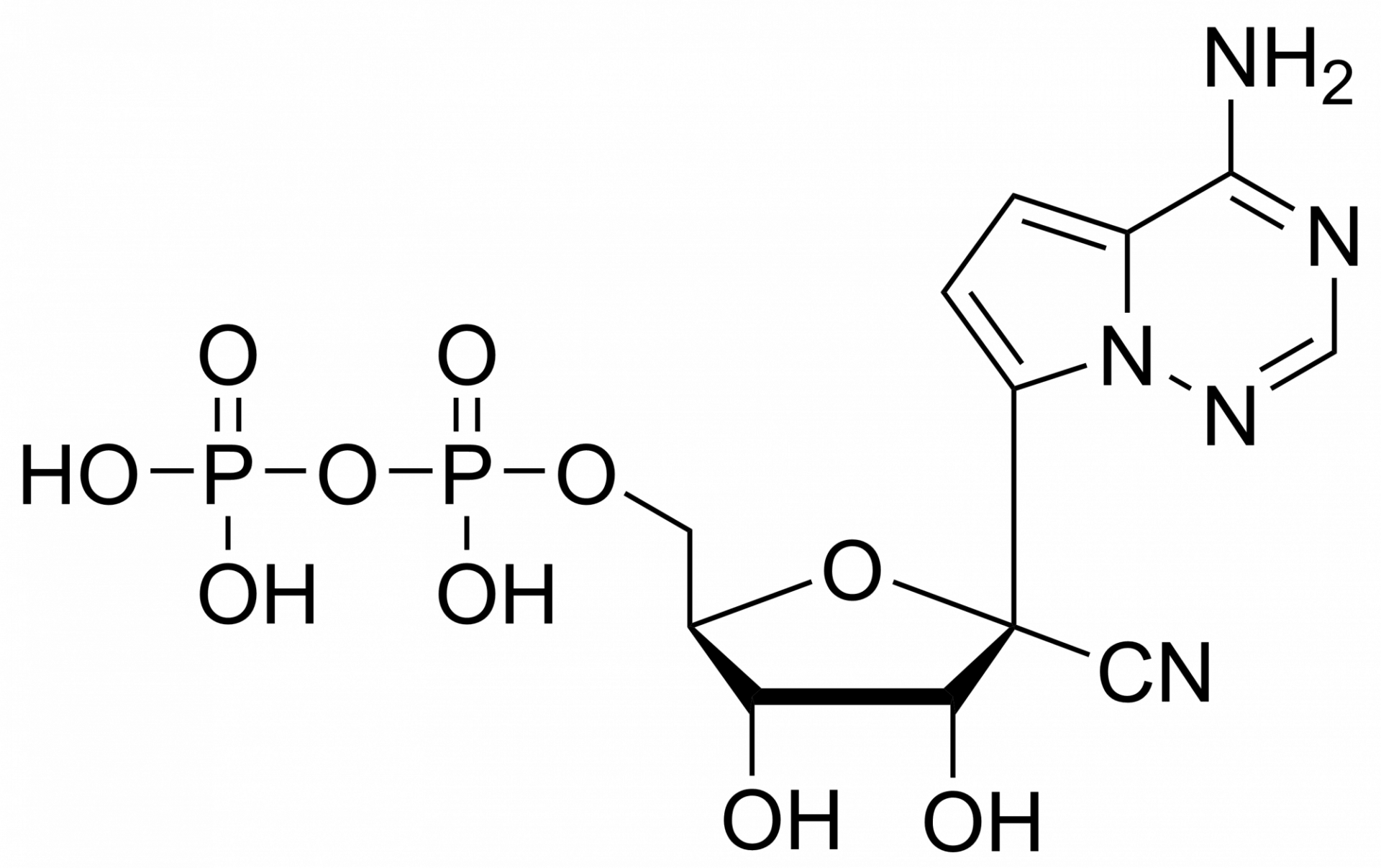
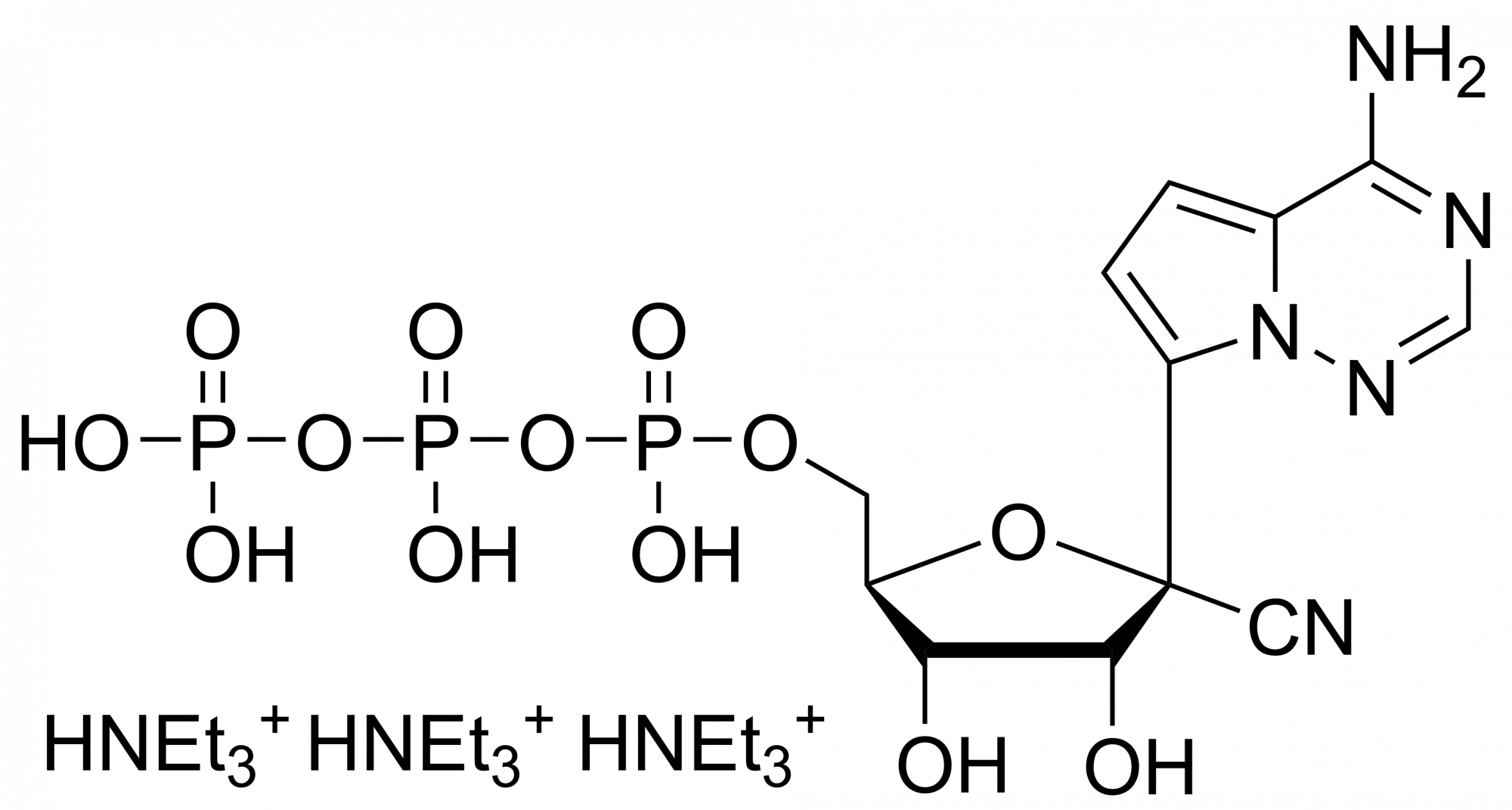
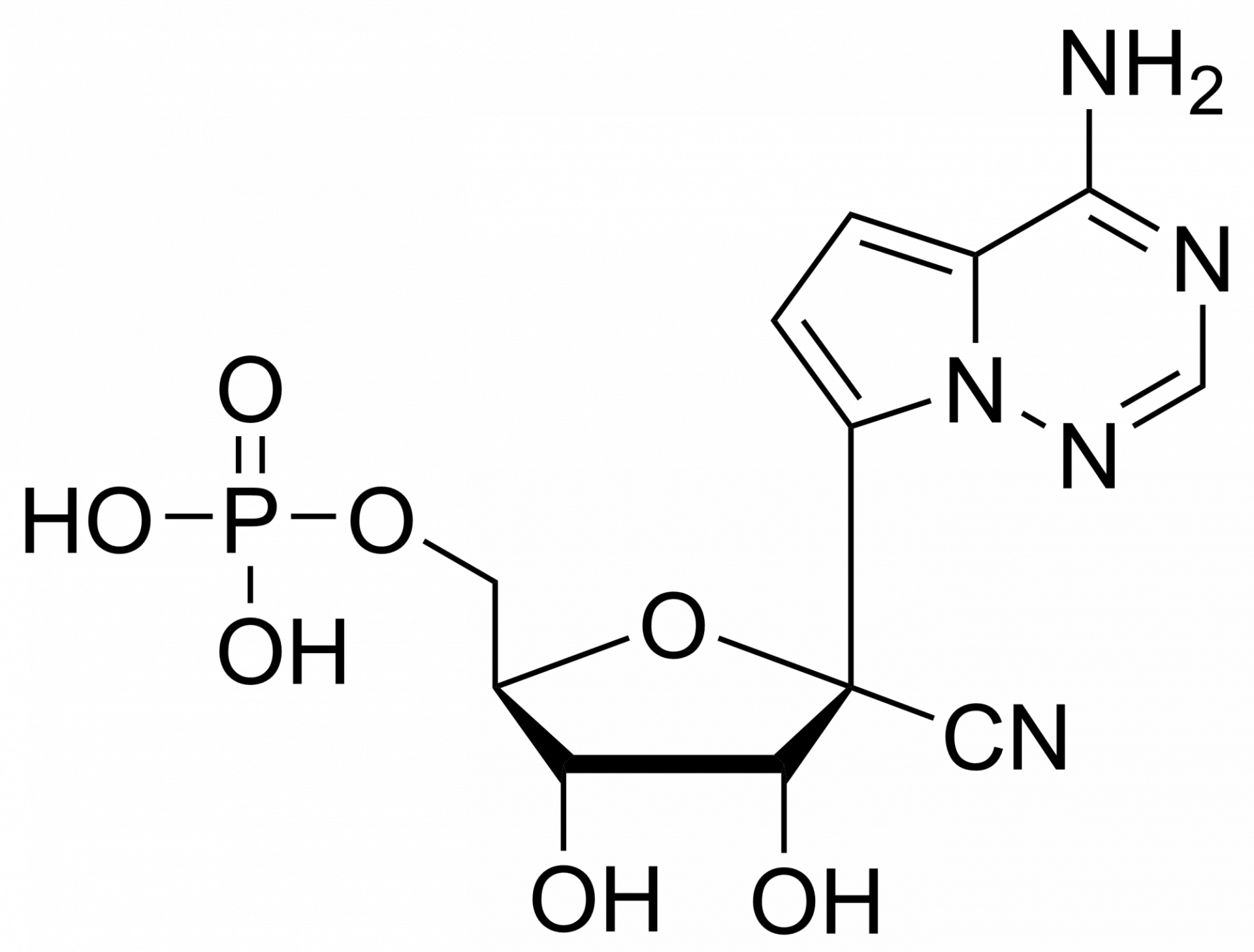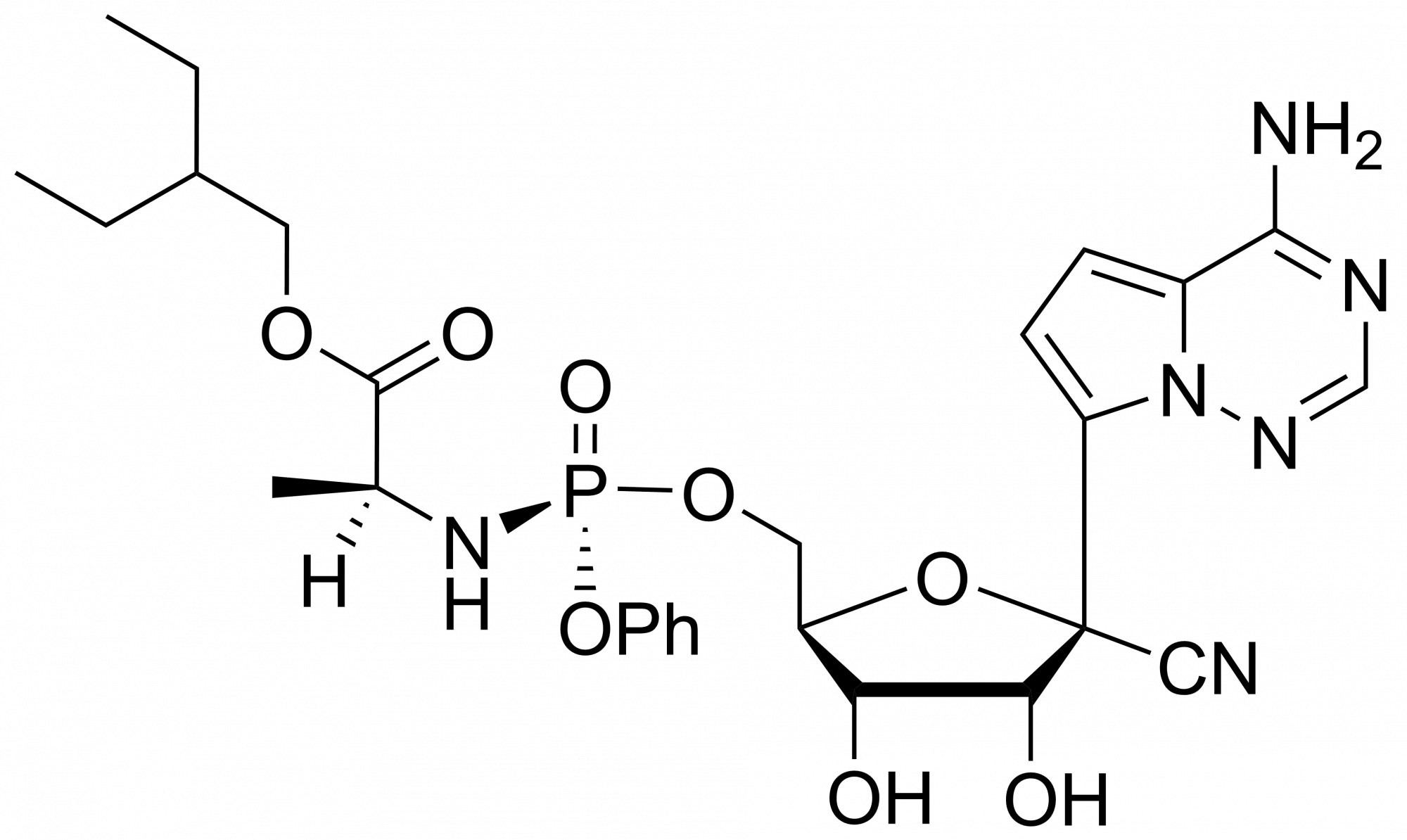Remdesivir triphosphate trisodium – GS-443902 – CAS 1355050-21-3
Remdesivir triphosphate trisodium – GS-443902 – CAS 1355050-21-3 is synthesised by Sigut Labs (Prague, Czech Republic).
We are also providing Remdesivir triphosphate in the form of Et3NH+ salt.
Purity (LC-MS)
99%+
Package contents
Remdesivir triphosphate trisodium salt
This compound is for research use only. We do not sell to patients.
| 1 mg | € 650 | Stock | ||
| 5 mg | € 780 | Stock | ||
| 10 mg | € 1115 | Stock | ||
| 25 mg | € 1960 | Stock | ||
| 50 mg | € 3220 | Stock | ||
| 100 mg | € 5090 | Stock | ||
| 100mM/10μL | € 650 | Stock | ||
| 10mM/100μL | € 650 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Stock
Stock
Stock
Stock
Stock
Stock
Characterisation
Description
Remdesivir triphosphate is synthesised by Sigut Labs (Prague, Czech Republic). GS-443902 is the active triphosphate metabolite of Remdesivir with activity against zoonotic feline infectious peritonitis virus (FIPV) and severe acute respiratory syndrome (SARS) virus from the Coronaviridae family. GS-443902 (GS-441524 triphosphate) is a potent viral RNA-dependent RNA-polymerases (RdRp) inhibitor with IC50s of 1.1 µM, 5 µM for RSV RdRp and HCV RdRp, respectively.
Remdesivir, developed by Gilead Sciences, is an adenosine prodrug that metabolizes into its active form GS-441524, which interferes with the action of viral RNA-dependent RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production.
This product is prepared and shipped as Na+ salt. If you are interested in Et3NH+ salt, visit our product page.
All products are lyophilized and can easily sustain the shipment oversee (shipping with DHL on dry ice to ensure that product arrives in a perfect state).
We are also offering this product as a water solution.
Chemicals are distributed worldwide
Buy Remdesivir triphosphate trisodium now, get your order in 48 hours.
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Shannon, A.; Le, N. T.-T.; Selisko, B.; Eydoux, C.; Alvarez, K.; Guillemot, J.-C.; Decroly, E.; Peersen, O.; Ferron, F.; Canard, B., Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020, 178, 104793.
- Ju, J.; Li, X.; Kumar, S.; Jockusch, S.; Chien, M.; Tao, C.; Morozova, I.; Kalachikov, S.; Kirchdoerfer, R. N.; Russo, J. J., Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020, 1-18.
- Jordan, P. C.; Liu, C.; Raynaud, P.; Lo, M. K.; Spiropoulou, C. F.; Symons, J. A.; Beigelman, L.; Deval, J., Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathogens 2018, 14 (2), e1006889/1-e1006889/23.
- Siegel, D.; Hui, H. C.; Doerffler, E.; Clarke, M. O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; Wang, Q.; Wolfe, L.; Jordan, R.; Soloveva, V.; Knox, J.; Perry, J.; Perron, M.; Stray, K. M.; Barauskas, O.; Feng, J. Y.; Xu, Y.; Lee, G.; Rheingold, A. L.; Ray, A. S.; Bannister, R.; Strickley, R.; Swaminathan, S.; Lee, W. A.; Bavari, S.; Cihlar, T.; Lo, M. K.; Warren, T. K.; Mackman, R. L., Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60 (5), 1648-1661.
- Clarke, M. O. N. H.; Jordan, R.; Mackman, R. L.; Ray, A. S.; Siegel, D. Preparation of amino acid-containing nucleosides for treating flaviviridae virus infections. 2017-US28243, 2017184668, 20170419., 2017.
- Warren, T. K.; Jordan, R.; Lo, M. K.; Ray, A. S.; Mackman, R. L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H. C.; Larson, N.; Strickley, R.; Wells, J.; Stuthman, K. S.; Van Tongeren, S. A.; Garza, N. L.; Donnelly, G.; Shurtleff, A. C.; Retterer, C. J.; Gharaibeh, D.; Zamani, R.; Kenny, T.; Eaton, B. P.; Grimes, E.; Welch, L. S.; Gomba, L.; Wilhelmsen, C. L.; Nichols, D. K.; Nuss, J. E.; Nagle, E. R.; Kugelman, J. R.; Palacios, G.; Doerffler, E.; Neville, S.; Carra, E.; Clarke, M. O.; Zhang, L.; Lew, W.; Ross, B.; Wang, Q.; Chun, K.; Wolfe, L.; Babusis, D.; Park, Y.; Stray, K. M.; Trancheva, I.; Feng, J. Y.; Barauskas, O.; Xu, Y.; Wong, P.; Braun, M. R.; Flint, M.; McMullan, L. K.; Chen, S.-S.; Fearns, R.; Swaminathan, S.; Mayers, D. L.; Spiropoulou, C. F.; Lee, W. A.; Nichol, S. T.; Cihlar, T.; Bavari, S., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature (London, United Kingdom) 2016, 531 (7594), 381-385.
- Chun, B. K.; Clarke, M. O. N. H.; Doerffler, E.; Hui, H. C.; Jordan, R.; Mackman, R. L.; Parrish, J. P.; Ray, A. S.; Siegel, D. Preparation of nucleosides and methods for treating Filoviridae virus infections. 2015-14926062 20160122374, 20151029., 2016.
- Cho, A.; Saunders, O. L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J. E.; Feng, J. Y.; Ray, A. S.; Kim, C. U., Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic & Medicinal Chemistry Letters 2012, 22 (8), 2705-2707.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors