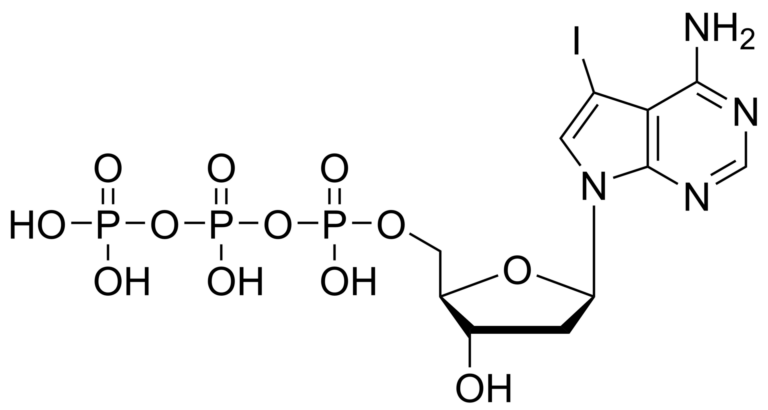
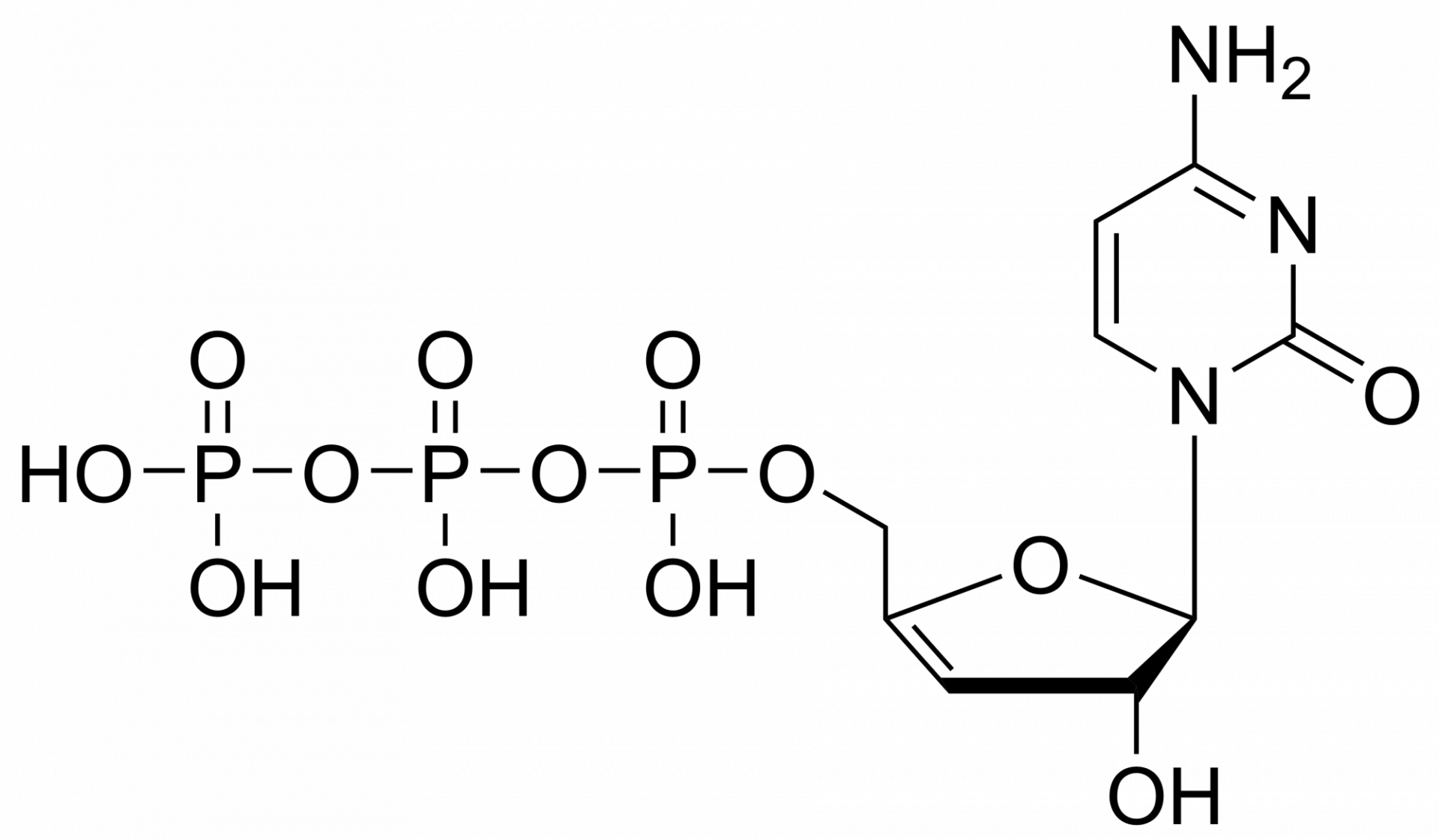
7-iodo-7-deaza-2′-deoxyadenosine triphosphate – CAS 214833-26-8
7-iodo-7-deaza-2′-deoxyadenosine triphosphate – CAS 214833-26-8 is synthesised by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
95%+
Package contents
7-iodo-7-deaza-2′-deoxyadenosine triphosphate Et3NH+ salt
This compound is for research use only. We do not sell to patients.
| 1 mg | € 500 | Stock | ||
| 5 mg | € 1600 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Characterisation
Description
7-iodo-7-deaza-2′-deoxyadenosine triphosphate is a metabolite of 7-iodo-7-deaza-2′-deoxyadenosine, a synthetic nucleotide analogue that is used as a probe for mapping RNA sequences and as an inhibitor of bacterial growth. This compound is also used in the synthesis of oligodeoxynucleotides because it has a high affinity for guanine residues, which can be incorporated into the oligonucleotide chain by chemical ligation. The octameric form of 7-deaza-2′-deoxyadenosine is most active against bacterial cells.
Chemicals are distributed worldwide
Buy 7-iodo-7-deaza-2′-deoxyadenosine triphosphate now, and get your order in 48 hours
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Seela, F., Feiling, E., Gross, J., Hillenkamp, F., Ramzaeva, N., Rosemeyer, H., & Zulauf, M. (2001). Fluorescent DNA: the development of 7-deazapurine nucleoside triphosphates applicable for sequencing at the single molecule level. Journal of Biotechnology, 86(3), 269-279.
- Vrábel, M., Pohl, R., Votruba, I., Sajadi, M., Kovalenko, S. A., Ernsting, N. P., & Hocek, M. (2008). Synthesis and photophysical properties of 7-deaza-2′-deoxyadenosines bearing bipyridine ligands and their Ru (II)-complexes in position 7. Organic & Biomolecular Chemistry, 6(16), 2852-2860.
- Čapek, P., Cahová, H., Pohl, R., Hocek, M., Gloeckner, C., & Marx, A. (2007). An efficient method for the construction of functionalized DNA bearing amino acid groups through cross‐coupling reactions of nucleoside triphosphates followed by primer extension or PCR. Chemistry–A European Journal, 13(21), 6196-6203.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors