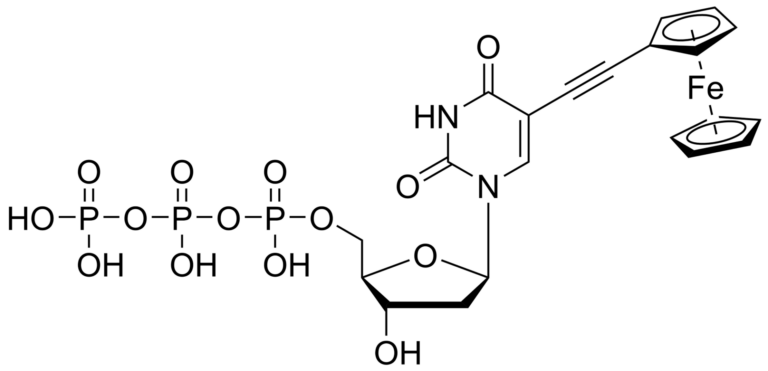
5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridinetriphosphate – CAS 1007229-84-6
5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridinetriphosphate – CAS 1007229-84-6 was synthesized and licensed at the IOCB Prague, lab of prof. Michal Hocek.
Purity (LC-MS)
99 %
Package contents
5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridine triphosphate Na+ salt
This compound is for research use only. We do not sell to patients.
| 1 mg | € 1400 | Stock | ||
| 5 mg | € 3500 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Characterisation
Description
5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridinetriphosphate is a ferrocene labeled analog of natural uridine triphosphate. Ferrocene is used as the gold standard for electrochemical labeling. Its conjugation at 5- position of uridine triphosphate allows enzymatic incorporation of such analog in primer extension reactions and PCR. The compound might be used for electrochemical redox labeling of DNA, site-specific redox labeling, or in the development of highly sensitive genosensors.
5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridine triphosphate was synthesized and licensed at the IOCB Prague, lab of prof. Michal Hocek.
Chemicals are distributed worldwide
Buy 5-(Ferrocene-1-yl-ethynyl)-2′-deoxyuridine triphosphate now, get your order in 48 hours.
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Brázdilová, P.; Vrábel, M.; Pohl, R.; Pivoňková, H.; Havran, L.; Hocek, M.; Fojta, M., Ferrocenylethynyl Derivatives of Nucleoside Triphosphates: Synthesis, Incorporation, Electrochemistry, and Bioanalytical Applications. Chemistry – A European Journal 2007, 13 (34), 9527-9533.
- Ménová, P.; Cahová, H.; Plucnara, M.; Havran, L.; Fojta, M.; Hocek, M., Polymerase synthesis of oligonucleotides containing a single chemically modified nucleobase for site-specific redox labelling. Chemical Communications 2013, 49 (41), 4652-4654.
- Magriñá, I.; Toldrà, A.; Campàs, M.; Ortiz, M.; Simonova, A.; Katakis, I.; Hocek, M.; O’Sullivan, C. K., Electrochemical genosensor for the direct detection of tailed PCR amplicons incorporating ferrocene labelled dATP. Biosensors and Bioelectronics 2019, 134, 76-82.
- Simonova, A.; Magriñá, I.; Sýkorová, V.; Pohl, R.; Ortiz, M.; Havran, L.; Fojta, M.; O’Sullivan, C. K.; Hocek, M., Tuning of Oxidation Potential of Ferrocene for Ratiometric Redox Labeling and Coding of Nucleotides and DNA. Chemistry – A European Journal 2020, 26 (6), 1286-1291.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors