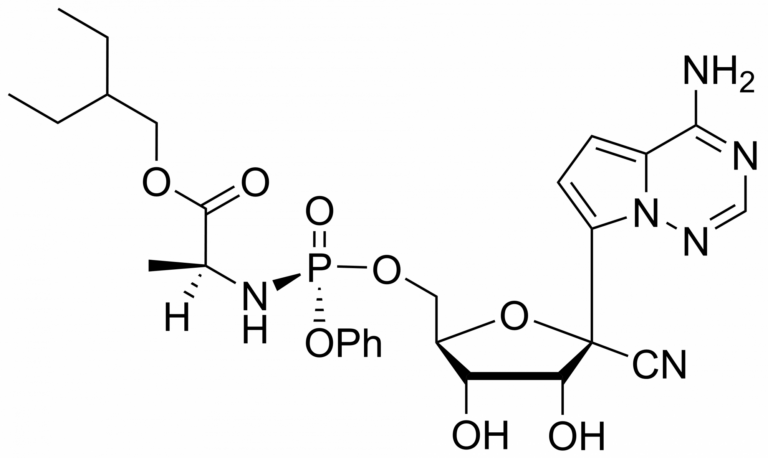
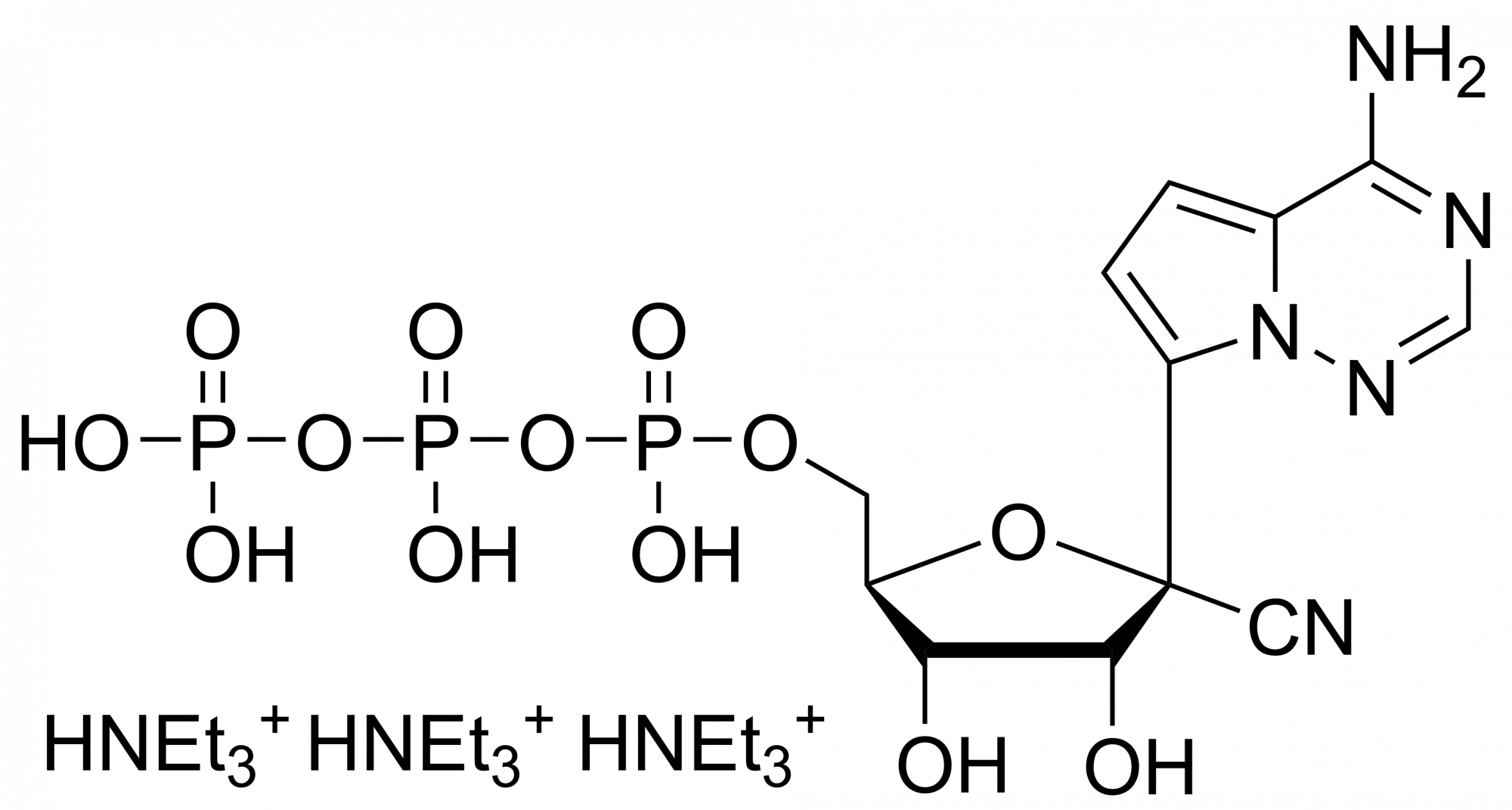
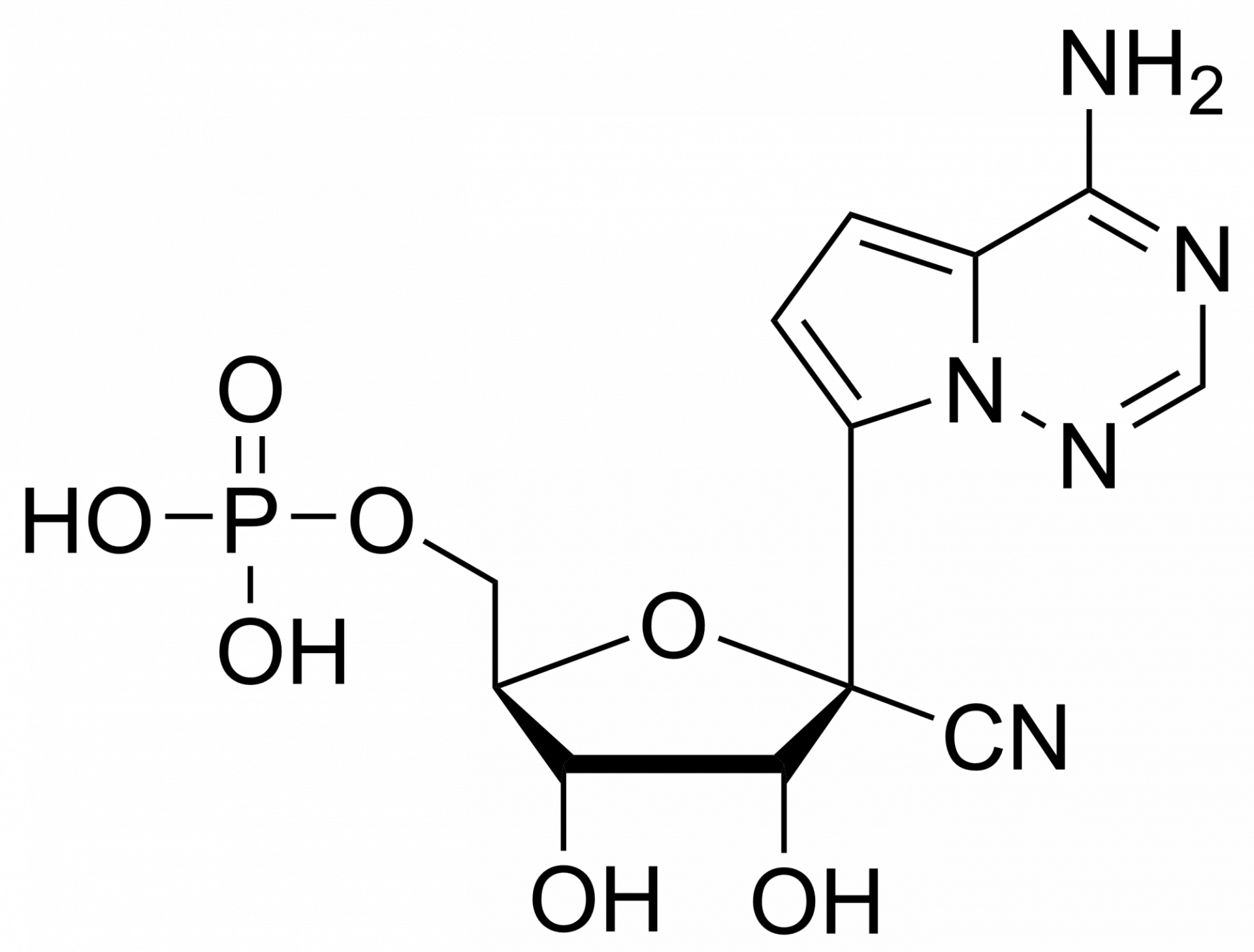Remdesivir – CAS 1809249-37-3
Remdesivir – CAS 1809249-37-3 is synthesised by Sigut Labs (Prague, Czech Republic).
Purity (LC-MS)
99%, d.e. 95%+
Package contents
Remdesivir
This compound is for research use only. We do not sell to patients.
| 50 mg | € 200 | Stock | ||
| 100 mg | € 250 | Stock | ||
| 1 g | € 450 | Stock | ||
| 5 g | € 700 | Stock | ||
| Do you want custom amount? | Custom amount | |||
Stock
Stock
Stock
Stock
Characterisation
Description
Remdesivir (also known as GS-5734) is synthesised by Sigut Labs (Prague, Czech Republic). It is an antiviral drug that belongs to the class of nucleotide analogues. It was mainly developed as a treatment for filovirus infections such as Ebola virus disease or Marburg virus. Subsequently, Remdesivir was found to show antiviral activity against other single-stranded RNA viruses such as respiratory syncytial virus, Nipah virus, Hendra virus, Junin virus, Lassa fever virus and the coronaviruses (including MERS and SARS viruses). Recently it is being studied as a promising medication for the treatment of COVID-19 (new SARS type virus).
Remdesivir, developed by Gilead Sciences, is an adenosine prodrug that metabolizes into its active form GS-441524 which interferes with the action of viral RNA-dependent RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production.
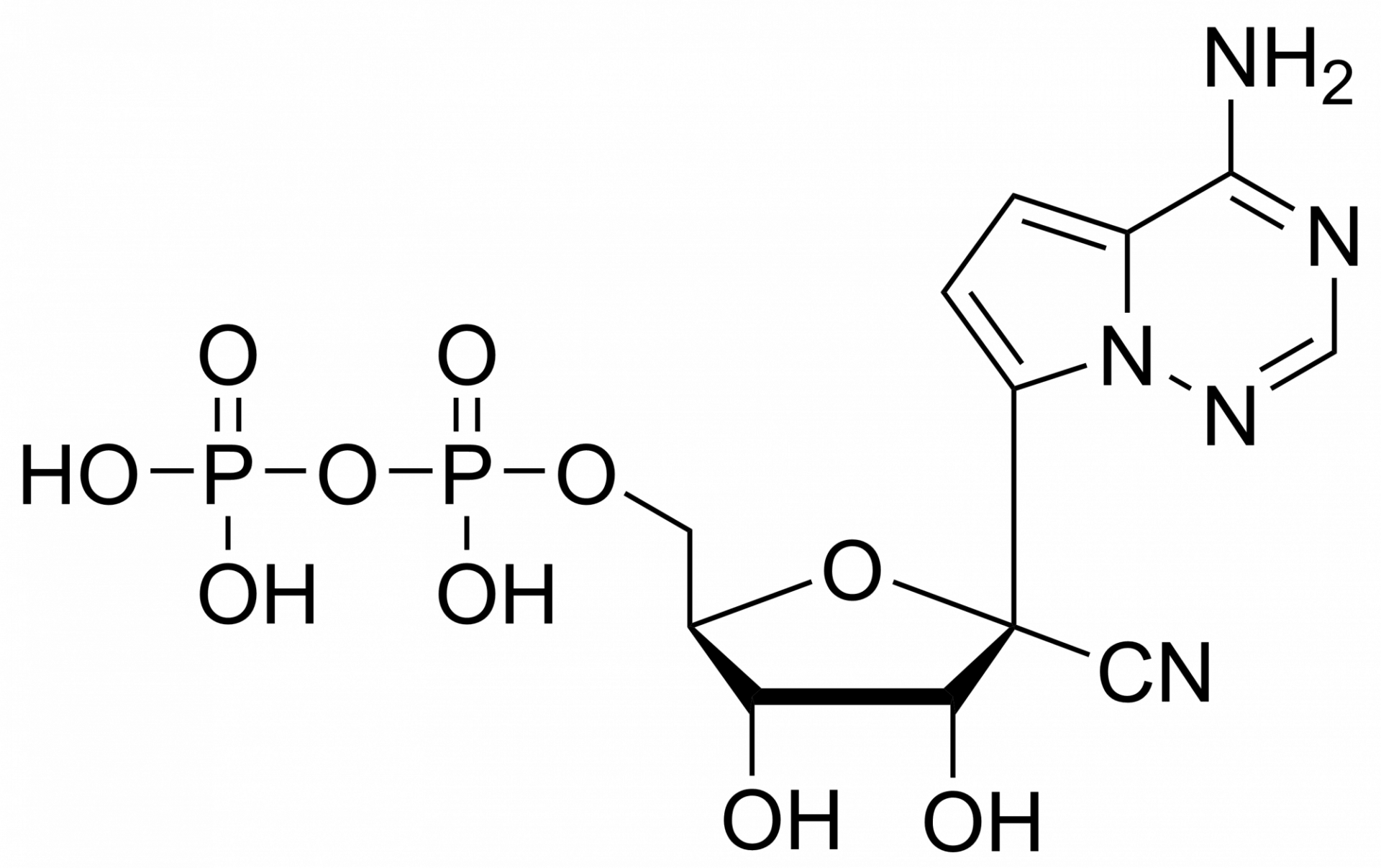
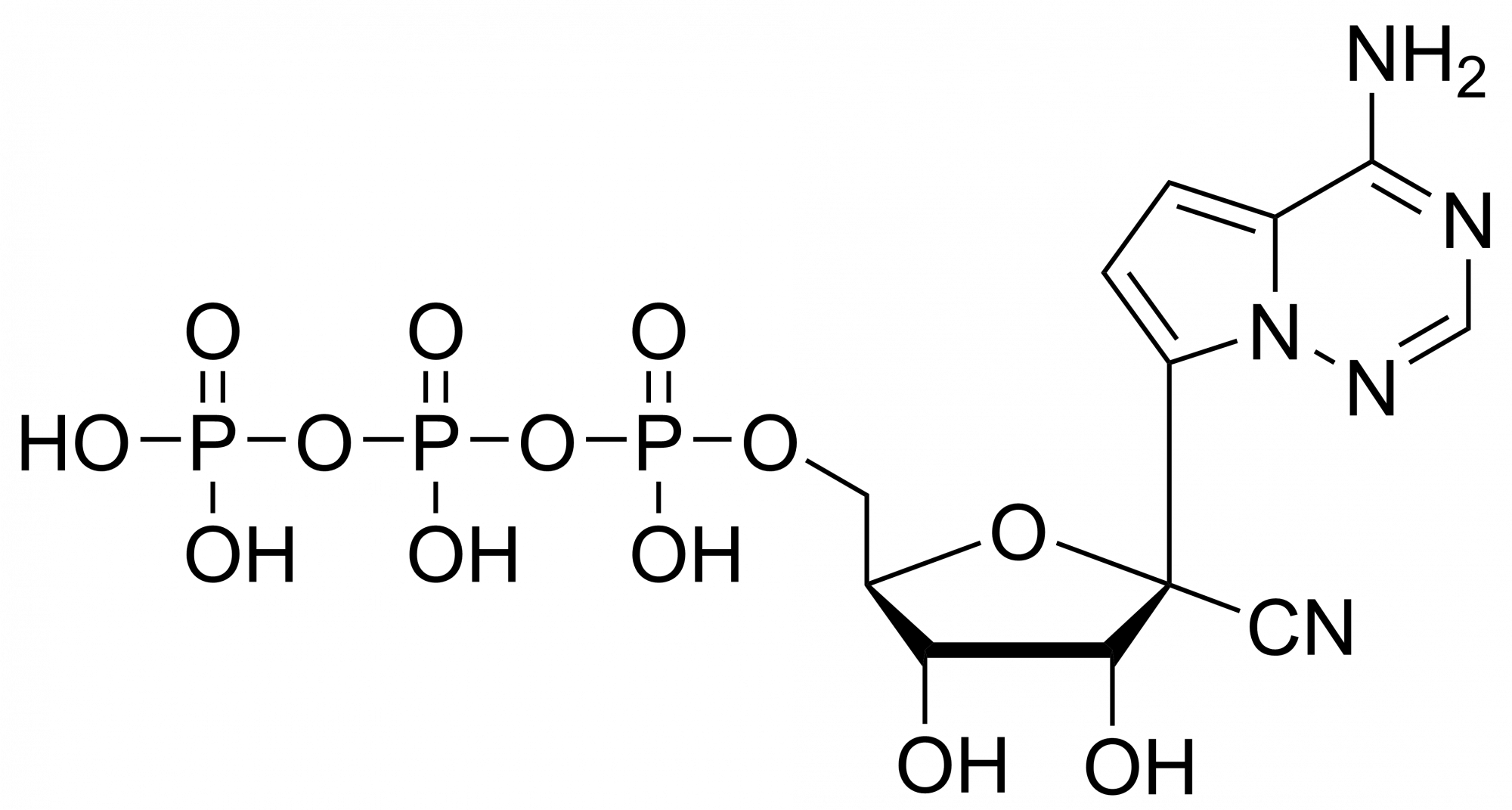
We are also offering active metabolites of Remdesivir: Remdesivir triphosphate, diphosphate or monophosphate.
Chemicals are distributed worldwide
Buy Remdesivir, get your order in 48 hours.
- Shipping through DHL in 48 hours
- Sensitive compounds are shipped on dry ice
- All compounds are safely and rigorously packed
Payment
- Payment terms 30 days net
- We are sending the invoice the same day as the shipment
- We are able to modify the invoice for the academic institution, so the order can be paid from grants
References
- Shannon, A.; Le, N. T.-T.; Selisko, B.; Eydoux, C.; Alvarez, K.; Guillemot, J.-C.; Decroly, E.; Peersen, O.; Ferron, F.; Canard, B., Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020, 178, 104793.
- Ju, J.; Li, X.; Kumar, S.; Jockusch, S.; Chien, M.; Tao, C.; Morozova, I.; Kalachikov, S.; Kirchdoerfer, R. N.; Russo, J. J., Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020, 1-18.
- Jordan, P. C.; Liu, C.; Raynaud, P.; Lo, M. K.; Spiropoulou, C. F.; Symons, J. A.; Beigelman, L.; Deval, J., Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathogens 2018, 14 (2), e1006889/1-e1006889/23.
- Siegel, D.; Hui, H. C.; Doerffler, E.; Clarke, M. O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; Wang, Q.; Wolfe, L.; Jordan, R.; Soloveva, V.; Knox, J.; Perry, J.; Perron, M.; Stray, K. M.; Barauskas, O.; Feng, J. Y.; Xu, Y.; Lee, G.; Rheingold, A. L.; Ray, A. S.; Bannister, R.; Strickley, R.; Swaminathan, S.; Lee, W. A.; Bavari, S.; Cihlar, T.; Lo, M. K.; Warren, T. K.; Mackman, R. L., Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60 (5), 1648-1661.
- Clarke, M. O. N. H.; Jordan, R.; Mackman, R. L.; Ray, A. S.; Siegel, D. Preparation of amino acid-containing nucleosides for treating flaviviridae virus infections. 2017-US28243, 2017184668, 20170419., 2017.
- Warren, T. K.; Jordan, R.; Lo, M. K.; Ray, A. S.; Mackman, R. L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H. C.; Larson, N.; Strickley, R.; Wells, J.; Stuthman, K. S.; Van Tongeren, S. A.; Garza, N. L.; Donnelly, G.; Shurtleff, A. C.; Retterer, C. J.; Gharaibeh, D.; Zamani, R.; Kenny, T.; Eaton, B. P.; Grimes, E.; Welch, L. S.; Gomba, L.; Wilhelmsen, C. L.; Nichols, D. K.; Nuss, J. E.; Nagle, E. R.; Kugelman, J. R.; Palacios, G.; Doerffler, E.; Neville, S.; Carra, E.; Clarke, M. O.; Zhang, L.; Lew, W.; Ross, B.; Wang, Q.; Chun, K.; Wolfe, L.; Babusis, D.; Park, Y.; Stray, K. M.; Trancheva, I.; Feng, J. Y.; Barauskas, O.; Xu, Y.; Wong, P.; Braun, M. R.; Flint, M.; McMullan, L. K.; Chen, S.-S.; Fearns, R.; Swaminathan, S.; Mayers, D. L.; Spiropoulou, C. F.; Lee, W. A.; Nichol, S. T.; Cihlar, T.; Bavari, S., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature (London, United Kingdom) 2016, 531 (7594), 381-385.
- Chun, B. K.; Clarke, M. O. N. H.; Doerffler, E.; Hui, H. C.; Jordan, R.; Mackman, R. L.; Parrish, J. P.; Ray, A. S.; Siegel, D. Preparation of nucleosides and methods for treating Filoviridae virus infections. 2015-14926062 20160122374, 20151029., 2016.
- Cho, A.; Saunders, O. L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J. E.; Feng, J. Y.; Ray, A. S.; Kim, C. U., Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic & Medicinal Chemistry Letters 2012, 22 (8), 2705-2707.
Similar products
Didn't find the chemical you were looking for?
Contact us
WHY CHOOSE
SigutLabs
Your impossible is our starting line
Partners & distributors